Metal-on-metal (MoM) hip arthroplasty was expected to provide benefits over metal-on-polyethylene systems. After widespread placement of MoM implants, outcomes have been disappointing. MoM implants are associated with higher serum levels of metal ions, adverse periarticular soft tissue reactions, and increased long-term failure rates. In light of these findings, it is crucial that patients with MoM implants be closely monitored for adverse effects. MR imaging is ideally suited for assessment of these patients and complements standard clinical evaluation and laboratory testing. This article reviews the background of MoM implants, emerging data on complications, strategies for using MR imaging, and MR imaging findings in patients with reaction to metal.
Key Points
- •
Metal-on-metal (MoM) implants are commonly used in total hip arthroplasty, with estimates of more than 1 million placed in the past 20 years.
- •
Compared with metal-on-polyethylene (MoPoly) implants, MoM implants are associated with elevated serum levels of metal ions, adverse periarticular soft tissue reactions, and increased long-term failure rates.
- •
In patients with suspected reaction to metal, MR imaging is most valuable when susceptibility artifact is minimized by careful selection and design of pulse sequences. Proton density (PD)-weighted images and T1-weighted images together enable soft tissue discrimination and the characterization of pseudotumors.
- •
In patients with reaction to metal, MR imaging findings most frequently include (1) juxta-articular collections that communicate with the joint space and (2) thickened periarticular soft tissues with irregular, infiltrative margins and low signal intensity.
- •
MR imaging complements clinical evaluation and laboratory testing in identification and monitoring of patients with reaction to metal and may help guide management including revision arthroplasty.
Introduction
Since their introduction in the 1960s as a system for total hip arthroplasty, MoPoly implants have demonstrated excellent long-term results, with some designs yielding approximately 80% implant survival at 25 years, averaged over all patient populations. In this system, the metal ball (cobalt-chrome) of the femoral component articulates with the plastic (polyethylene) liner of the acetabular component. Although there are many reasons for hardware failure, such as infection, loosening, and dislocation, longevity of the MoPoly implant is limited primarily by polyethylene wear, periprosthetic osteolysis caused by the release of plastic debris into the joint, and aseptic loosening. In older patients, the expected lifespan of MoPoly is usually satisfactory. In younger patients, however, implant durability is decreased, likely because of higher activity levels, and implant longevity begins to drop well below that reported in the older population. Given longer life expectancy in the younger population, prosthetic implant durability and osteolysis represent both important preoperative considerations in the choice of implant and potential postoperative complications requiring revision arthroplasty.
To combat wear of the plastic liner, other bearing combinations besides MoPoly have been tested, including ceramic ball in ceramic socket and metal ball in metal socket. By eliminating the plastic liner and using hard-on-hard bearing surfaces, the expectation is that implant durability can be extended.
Compared with MoPoly, MoM arthroplasty has demonstrated lower in vitro wear rates. Two major MoM designs have been developed. In one of these, the implant maintains a conventional design, including a complete femoral component. In the other, the acetabular component is the same but the femoral head is resurfaced rather than removed entirely. In this resurfacing arthroplasty, the stem or peg of the femoral component is short and extends only into the femoral neck, thereby preserving bone. Potential benefits of resurfacing include increased postoperative activity, range of motion, joint stability, and the possibility of more successful revision surgery due to the preservation of the femoral neck. Due to the theoretic superiority and initial promising results with both resurfacing and total hip arthroplasty, the use of MoM implants rose steadily. The National Joint Registry (NJR) for England and Wales recorded in 2008–2009 that MoM resurfacing procedures represented 30% of primary hip arthroplasties in patients less than 55 years of age. In the United States, review of the Nationwide Inpatient Sample database from 2005–2006 revealed that 35% of all total hip replacements used MoM bearings, including 69% of replacements in patients under 65 years of age. One group estimated that more than 1 million MoM implants have been placed over the past 2 decades.
Despite early success, more recent data have revealed higher than expected rates of failure after MoM arthroplasty. In 2010–2011, the NJR of England and Wales showed a mean 7-year revision rate of 13.6% for MoM total hip replacement and 11.3% for MoM resurfacing hip replacement compared with 3.4% for MoPoly total hip replacement. The National Joint Replacement Registry in Australia recorded a 7.3% revision rate at 7 years after MoM arthroplasty compared with a 4.8% revision rate after MoPoly arthroplasty. Another report suggested that revision rates and even re-revision rates were higher after MoM resurfacing. Based on data from the NJR of England and Wales, Smith and colleagues concluded that all patients with MoM implants should be carefully monitored for failure. In this same study, the investigators recommended that ceramic-on-ceramic bearings could be continued, but MoM articulations should not be implanted in the future. Current recommendations from the US Food and Drug Administration and the UK Medicines and Healthcare Products Regulatory Agency include restricted use of MoM prostheses and careful monitoring of all patients who have received MoM hip arthroplasty.
In light of these unexpected outcomes, several investigators have attempted to elucidate the reasons for the increased failure rate. The evidence to date indicates that multiple factors are involved, including the design and composition of the bearing surfaces, the alignment of the components achieved by the surgeon at the time of implantation, and the degree of patient-specific sensitivity to metal. Although each factor contributes variably to the development of pathology in an individual patient, the combined effect on local tissues contributes substantially to the increased failure rate. Local reaction to metal involves predominantly periprosthetic soft tissues and, as experience has grown, has been described by different terms in the literature. Most commonly, the soft tissue reaction to metal has been called pseudotumor , a borrowed term originally used to describe solid masses resulting from polyethylene wear debris in patients with MoPoly arthroplasty and osteolysis. This review continues to use the generic term, pseudotumor, to encompass the spectrum of local soft tissue abnormalities that reflect reaction to metal. In the setting of MoM arthroplasty, pseudotumor is more likely to refer to complex cystic collections and periprosthetic soft tissue thickening rather than actual solid masses. Osteolysis, similar to that seen with MoPoly components, rarely occurs after MoM arthroplasty.
Introduction
Since their introduction in the 1960s as a system for total hip arthroplasty, MoPoly implants have demonstrated excellent long-term results, with some designs yielding approximately 80% implant survival at 25 years, averaged over all patient populations. In this system, the metal ball (cobalt-chrome) of the femoral component articulates with the plastic (polyethylene) liner of the acetabular component. Although there are many reasons for hardware failure, such as infection, loosening, and dislocation, longevity of the MoPoly implant is limited primarily by polyethylene wear, periprosthetic osteolysis caused by the release of plastic debris into the joint, and aseptic loosening. In older patients, the expected lifespan of MoPoly is usually satisfactory. In younger patients, however, implant durability is decreased, likely because of higher activity levels, and implant longevity begins to drop well below that reported in the older population. Given longer life expectancy in the younger population, prosthetic implant durability and osteolysis represent both important preoperative considerations in the choice of implant and potential postoperative complications requiring revision arthroplasty.
To combat wear of the plastic liner, other bearing combinations besides MoPoly have been tested, including ceramic ball in ceramic socket and metal ball in metal socket. By eliminating the plastic liner and using hard-on-hard bearing surfaces, the expectation is that implant durability can be extended.
Compared with MoPoly, MoM arthroplasty has demonstrated lower in vitro wear rates. Two major MoM designs have been developed. In one of these, the implant maintains a conventional design, including a complete femoral component. In the other, the acetabular component is the same but the femoral head is resurfaced rather than removed entirely. In this resurfacing arthroplasty, the stem or peg of the femoral component is short and extends only into the femoral neck, thereby preserving bone. Potential benefits of resurfacing include increased postoperative activity, range of motion, joint stability, and the possibility of more successful revision surgery due to the preservation of the femoral neck. Due to the theoretic superiority and initial promising results with both resurfacing and total hip arthroplasty, the use of MoM implants rose steadily. The National Joint Registry (NJR) for England and Wales recorded in 2008–2009 that MoM resurfacing procedures represented 30% of primary hip arthroplasties in patients less than 55 years of age. In the United States, review of the Nationwide Inpatient Sample database from 2005–2006 revealed that 35% of all total hip replacements used MoM bearings, including 69% of replacements in patients under 65 years of age. One group estimated that more than 1 million MoM implants have been placed over the past 2 decades.
Despite early success, more recent data have revealed higher than expected rates of failure after MoM arthroplasty. In 2010–2011, the NJR of England and Wales showed a mean 7-year revision rate of 13.6% for MoM total hip replacement and 11.3% for MoM resurfacing hip replacement compared with 3.4% for MoPoly total hip replacement. The National Joint Replacement Registry in Australia recorded a 7.3% revision rate at 7 years after MoM arthroplasty compared with a 4.8% revision rate after MoPoly arthroplasty. Another report suggested that revision rates and even re-revision rates were higher after MoM resurfacing. Based on data from the NJR of England and Wales, Smith and colleagues concluded that all patients with MoM implants should be carefully monitored for failure. In this same study, the investigators recommended that ceramic-on-ceramic bearings could be continued, but MoM articulations should not be implanted in the future. Current recommendations from the US Food and Drug Administration and the UK Medicines and Healthcare Products Regulatory Agency include restricted use of MoM prostheses and careful monitoring of all patients who have received MoM hip arthroplasty.
In light of these unexpected outcomes, several investigators have attempted to elucidate the reasons for the increased failure rate. The evidence to date indicates that multiple factors are involved, including the design and composition of the bearing surfaces, the alignment of the components achieved by the surgeon at the time of implantation, and the degree of patient-specific sensitivity to metal. Although each factor contributes variably to the development of pathology in an individual patient, the combined effect on local tissues contributes substantially to the increased failure rate. Local reaction to metal involves predominantly periprosthetic soft tissues and, as experience has grown, has been described by different terms in the literature. Most commonly, the soft tissue reaction to metal has been called pseudotumor , a borrowed term originally used to describe solid masses resulting from polyethylene wear debris in patients with MoPoly arthroplasty and osteolysis. This review continues to use the generic term, pseudotumor, to encompass the spectrum of local soft tissue abnormalities that reflect reaction to metal. In the setting of MoM arthroplasty, pseudotumor is more likely to refer to complex cystic collections and periprosthetic soft tissue thickening rather than actual solid masses. Osteolysis, similar to that seen with MoPoly components, rarely occurs after MoM arthroplasty.
Adverse reaction to metal
In patients with MoM implants, the literature currently supports 2 main mechanisms to explain adverse reaction to metal:
- 1.
Excessive wear of bearing surfaces causing metallic debris and periprosthetic soft tissue deposition (dose-dependent reaction depending on the degree of metal wear)
- 2.
Hypersensitivity to metal (reaction to metal does not require substantial wear of bearing surfaces)
Excessive wear of bearing surfaces depends on several factors, such as component composition, corrosion, and abnormal loading across the implant surfaces. At revision surgery in patients with pseudotumors, retrieved implants frequently demonstrate excessive metal wear along the weight-bearing edge of the acetabular component, indicating uneven load distribution that may result from component malposition (such as increased acetabular abduction/inclination or poor acetabular anteversion) and/or decreased head diameter. Excessive wear is associated with elevated cobalt and chromium levels in serum, red blood cells, urine, and joint fluid. Animal studies have demonstrated synovial ulceration, necrosis, macrophage infiltration, and lymphocyte response following a single injection of intra-articular cobalt and chromium with effects lasting up to two years. Although MoM failure rates seem to reflect dose-dependent reaction to metal ions, elevated serum levels of cobalt and chromium are not adequate for the diagnosis of implant failure, demonstrating a sensitivity of 63% and specificity of 86% in predicting which patients will present with implant failure and requirement for revision surgery.
Based on the histopathologic appearance of periarticular soft tissue samples retrieved from MoM patients, pathologists have described an aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL), which seems to represent an immune-mediated type IV delayed hypersensitivity reaction, at least locally. This phenomenon suggests that a subpopulation of patients has hypersensitivity to metal and, therefore, is more susceptible to the failure of MoM arthroplasties. In support of this immune-mediated etiology, some patients with failed implants demonstrate appropriate positioning and alignment of components, nonpathologic levels of cobalt and chromium, and minimal wear of retrieved components when evaluated postoperatively.
Other factors correlating with MoM implant failure include (1) gender: female patients demonstrate a higher risk of implant failure ; (2) size of the femoral ball: smaller femoral head sizes correlate with increased failure rates because of concentrated load and compromised wear properties compared with larger head sizes ; and (3) age: patients less than 40 years of age have increased rates of revision likely due to higher levels of activity.
In patients presenting with adverse reaction to metal, clinical symptoms are often nonspecific. Most frequently, lateral hip pain or groin discomfort is present. Compressive neurovascular symptoms may result from a palpable mass. Because MoM implants may develop infection and loosening similar to MoPoly implants, the work-up should take into account the possibility of other complications besides reaction to metal. Although some inflammatory markers may be elevated in patients with adverse reactions to metal, normal or low erythrocyte sedimentation rate and C-reactive protein levels help exclude infection. Imaging plays an important early role in symptomatic patients with MoM implants and suspected reaction to metal. As experience grows, MR imaging is more frequently requested as a baseline study even in asymptomatic patients with MoM arthroplasties. Fig. 1 shows an algorithm proposed for the work-up, surveillance, and management of patients with MoM implants (see Fig. 1 ).
The imaging armamentarium can include several modalities, depending on local resources, preferences, and expertise. Radiographs demonstrate the alignment of components, osteolysis, fracture, and loosening. Subsidence and other positional changes can be followed accurately over time. Component orientation, including acetabular inclination, acetabular version, and femoral stem or peg position, can be better quantitated using CT or radiographic views that are carefully standardized. Although radiographs often show the complications of particle disease in MoPoly implants, they are typically unrevealing in MoM implants despite substantial adverse tissue reaction. Whereas particle disease is characterized by osteolysis, reaction to metal is characterized by pseudotumor and other soft tissue abnormalities that are undetectable by radiographic techniques. Ultrasound can demonstrate superficial fluid collections and soft tissues masses in reaction to metal, but the true extent of involvement can be impossible to gauge because of pathologic tissue distortion and spread of tissue reaction into deep, poorly visualized compartments of the thigh and pelvis. Recent data suggest that ultrasound underestimates the prevalence of adverse tissue reaction. CT is more commonly requested in the assessment of periprosthetic osteolysis in MoPoly arthroplasty than for the assessment of periarticular pseudotumor in MoM arthroplasty.
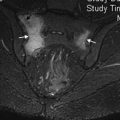
MR imaging has an established role in the evaluation of patients with suspected reaction to metal despite severe susceptibility artifact created by the ferromagnetic properties of metallic implants. MR imaging effectiveness depends on the limitation of this artifact. Diagnostic images are more challenging to produce in MoM implants compared with MoPoly prostheses due to the increased metal mass and corresponding susceptibility ( Fig. 2 ).
Imaging technique
At the authors’ institution, the MoM MR imaging protocol includes 5 sequences. First, short tau inversion recovery (STIR) coronal and T1-weighted axial sequences are obtained through the entire pelvis. Subsequently, PD-weighted images are prescribed with a smaller field-of-view in 3 planes through the affected hip. To limit the susceptibility artifact from metal, routine MR sequences and parameters must be modified. Specific parameters are discussed later ( Table 1 ).
| Sequence | Plane | TR | TE | FOV | Matrix | Slice (mm) | Skip (mm) | NEX | Echo Train | TI | Bandwidth |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1W TSE | Pelvis axial | 500–600 | 22 | 240 × 340 | 512 × 216 | 5 | 20% | 3–4 | 6 | — | 350–400 |
| STIR TSE | Pelvis coronal | 4100–4500 | 24 | 340 × 340 | 256 × 205 | 5 | 20% | 2 | 9 | 150 | 200–300 |
| PD TSE | Hip axial | 4200–4500 | 34 | 200 × 200 | 512 × 307 | 4 | 25% | 3 | 20 | — | 350–400 |
| PD TSE | Hip coronal | 3200–5400 | 34 | 200 × 200 | 512 × 359 | 4 | 25% | 1 | 20 | — | 350–400 |
| PD TSE | Hip sagittal | 3200–3400 | 34 | 200 × 200 | 512 × 359 | 4 | 25% | 3 | 20 | — | 350–400 |
Susceptibility Artifact
Magnetic susceptibility (or magnetizability) refers to the extent to which a material becomes magnetized when placed in an external magnetic field. Materials placed in a high-strength magnetic field (B 0 ) react to the effects of B 0 based on their molecular and atomic structure. Ferromagnetic materials, such as those used in orthopedic implants, have the capacity for concentrating and distorting local magnetic forces. Local field inhomogeneities created by a ferromagnetic material result in
- 1.
Dephasing of adjacent proton spins and signal decay through T2* effect
- 2.
Regional decrease or increase in local precessional frequencies causing erroneous spatial mapping of spins in the readout gradient, ultimately leading to signal dropout in one location and signal pileup in another location
- 3.
Shifts in local phase and frequency gradients and signals, resulting in geometric distortion along both the phase and frequency encoding axes.
Susceptibility Artifact Reduction
Standard commercial hardware and software allow pulse sequence manipulations that reduce susceptibility artifact. Several considerations should be made in the design of a MoM protocol:
- 1.
Susceptibility artifact is proportional to B 0 . Therefore, low-field systems may have an inherent advantage in the imaging of patients with bulk metal prostheses. Due to the predominance of 1.5-T magnets, a majority of MoM scans are performed using these systems. The parameters listed in this article apply to 1.5-T systems. Ultra–high-field magnets, such as 3-T units, should be avoided.
- 2.
Spin-echo sequences are less affected by susceptibility artifact because the 180° refocusing pulse mitigates spin dephasing. Gradient-echo sequences have limited or no role in MoM imaging.
- 3.
To characterize the T1-weighted signal from structures, the repetition time (TR) should remain in the range appropriate for T1-weighted images (500–600 ms at 1.5 T).
- 4.
In fast (turbo) spin-echo sequences, echo train length should be increased, although the trade-off is increased blurring artifact.
- 5.
By minimizing echo time (TE) and echo spacing, there is less time for spin dephasing and the associated loss in signal. The TE in T1-weighted sequences should be lower than 25 ms. PD-weighted sequences (TE <35 ms) are used instead of T2-weighted sequences due to decreased susceptibility artifact and superior anatomic detail.
- 6.
By increasing receiver bandwidth (BW), TE and echo spacing can be decreased. At 1.5-T, BW between 350 and 400 Hz/pixel balances the advantages of decreased susceptibility artifact with the disadvantages of decreased signal-to-noise ratio (SNR).
- 7.
Steeper amplitude of the frequency-encoding gradient diminishes the relative impact of field inhomogeneities.
- 8.
STIR sequences can be used as a fluid-sensitive, fat-suppression technique. Water excitation sequences may also have a role in MoM imaging. Frequency-selective (spectral) fat suppression is suboptimal because local field inhomogeneities prevent homogeneous fat saturation and often lead to the suppression of signal from tissues containing water rather than tissues containing fat.
- 9.
Decreasing voxel size limits diffusion-related signal loss and, by improving the spatial resolution, also improves delineation of the artifact. The trade-off is lower SNR. Parameter modifications include higher matrix (optimally >256 × 256), thinner slices, and smaller field-of-view.
Several investigators have proposed metal artifact reduction (MAR) imaging protocols. In MoM hip implants, the MR parameters listed in Table 1 are appropriate in any generic 1.5-T system to reduce susceptibility artifact and generate a diagnostic clinical study within a reasonable time frame (see Table 1 ):
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree