In the absence of distant metastatic disease, breast cancer staging is based on the extent of local-regional disease in the breast and axilla. The American Joint Committee on Cancer TNM system is used to provide breast cancer patients and their clinicians criteria for determining prognosis and treatment options. The TNM system categorizes extent of disease using anatomic data from the primary tumor (T), regional lymph nodes (N), and distant metastases (M) ( Tables 9-1 through 9-4 ). Clinical staging is performed initially, then replaced by pathologic staging following complete resection of the primary tumor and lymph node sampling. The use of neoadjuvant treatments (medical treatment prior to surgery) requires accurate preoperative clinical staging. Additionally, although not always altering the TNM stage, the extent of in-breast disease is critical to surgical planning.
Before the modern era of breast magnetic resonance imaging (MRI), clinical staging and surgical treatments were based primarily on the clinical exam, mammography, and ultrasound. The ability of MRI to identify additional, otherwise occult disease in the breast has added increased sensitivity and complexity. Imaging findings supplemented with image-guided biopsy of the breast and suspicious lymph nodes are central to determining clinical stage. Imaging findings must be collected within 4 months of diagnosis and in the absence of disease progression in order to be considered for clinical staging. Advances in breast cancer biologic markers (estrogen receptor [ER]/progesterone receptor [PR]/ human epidermal growth factor receptor 2[HER2]), multigene expression assays, and a better understanding of cancer biology may foretell prognosis and guide treatment more accurately than the solely anatomically-based TNM system. Today, treatment of newly diagnosed breast cancer incorporates both cancer biology and the TNM staging system.
Breast MRI is used to complement conventional mammographic and sonographic imaging for preoperative clinical staging and for surgical treatment planning based on the extent of disease in the ipsilateral breast. MRI provides a more accurate assessment of tumor size and distribution than physical exam, mammography, or ultrasound alone. When MRI is combined with these, disease-extent sensitivity of 99% can be obtained. A recent study found that 94% of radiology practices surveyed used MRI to evaluate the extent of known malignant disease before definitive treatment. In this chapter, the assessment for extent of disease in patients with newly diagnosed breast cancer will be reviewed. This includes evaluation of the ipsilateral breast for the size and extent of the known (index) tumor; multifocal and multicentric malignancy; involvement of the skin, nipple, pectoralis muscle and chest wall; evaluation for regional metastatic lymph node involvement; and evaluation of the contralateral breast for occult synchronous malignancy. Additionally, breast cancer may also arise in unique forms when the primary breast lesion is unknown. This includes metastatic disease in the axilla with an unknown primary malignancy, Paget disease of the nipple, and inflammatory breast carcinoma. MRI is also used in evaluating breast cancer patients undergoing neoadjuvant treatment and in those whose tumors were found to have positive surgical margins after breast conserving surgery.
Evaluation of the Ipsilateral Breast
Index Tumor Size
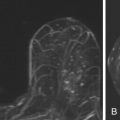
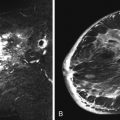
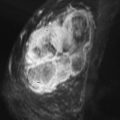
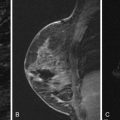
Local tumor extent is classified into three types: unifocal, multifocal, and multicentric. Unifocal type is defined as only one malignant focus identified in the breast. Multifocal type is more than one malignant focus identified within the same quadrant of the breast. Multicentric type is greater than one malignant focus identified in multiple quadrants of the breast or separated by more than 4 cm. Multicentric disease is a contraindication to breast conservation therapy ( Figures 9.1 and 9.2 ).


The goal of breast MRI is to characterize the size of the known index malignancy and identify additional sites of occult malignancy to guide surgical planning. MRI demonstrates improved accuracy in determining pathologic tumor size over breast exam and conventional imaging. However, both overestimation and underestimation of disease do occur, and it has not yet been defined in which cases this is most likely to happen. There is limited evidence suggesting that improved correlation between tumor size and pathologic measurement occurs with both high-grade invasive tumors and high-grade ductal carcinoma in situ (DCIS) and underestimation of disease may occur more often with low-grade invasive tumors and low-grade DCIS. Also, there is a greater tendency for tumor size overestimation for tumors larger than 2 cm in size than for tumors smaller than 2 cm.
Multifocal and Multicentric Disease
Multifocal and/or multicentric malignancy may be present at the time of diagnosis but undetected by conventional mammographic and sonographic imaging. This may consist of one or more discrete enhancing masses separate from the known index tumor and/or nonmasslike enhancement, typical for DCIS ( Figures 9.3 and 9.4 ). The preoperative identification of additional sites of malignancy in the breast provides information for surgical planning. It has been consistently shown that breast MRI detects additional unsuspected malignancy within the ipsilateral breast. A recent meta-analysis of 50 studies found that MRI identified occult multicentric or multifocal malignancy in 20% of preoperative MRIs with a positive predictive value (PPV) of 75% and accuracy of 93% when using at least 1.5 Tesla (T) MRI. The same meta-analysis examined 26 studies (n = 4839) that gave information about surgical impact. They found an appropriate change in surgical management occurred in 12.8% of patients with confirmed additional malignancy, 8.3% of whom were converted to receive mastectomy and 4.5% of whom were converted to receive a wider local excision. On the other hand, overestimation of disease (false positives) resulted in inappropriate alteration in surgical management in 6.3% of patients, 4.6% of whom had a wider local excision and 1.7% of whom had mastectomies. The importance of not subjecting patients to unnecessary extensive surgery, combined with the known risk of overestimation of disease with MRI, emphasizes the need to obtain histologic confirmation of suspicious MRI findings before changing surgical treatment. This was not done in all of the studies included in the meta-analysis. Given a PPV of 75%, one in four patients with suspicious findings will, in fact, have benign disease. Additionally, given the imperfect specificity of MRI and limited data regarding the appropriate use of the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) category 3 in MRI, it is generally not acceptable to recommend short-term follow-up MRI imaging for suspicious lesions in the ipsilateral breast that may alter surgical treatment. Proceeding with an image-guided biopsy is often prudent in these cases.


Several studies have shown that diffusion-weighted imaging (DWI) and spectroscopy have shown promise in increasing the PPV of MRI. Further studies on these techniques are warranted.
False negatives, in which additional tumor is present but not detected, are also known to occur with MRI. Background diffuse parenchymal enhancement of normal tissue obscuring lesion enhancement is a common reason for false-negative imaging. Moderate and marked background enhancement can reduce the sensitivity for multifocal and multicentric lesions that are small and lack distinguishing morphologic features. Even extensive nonmasslike enhancement, which occurs with DCIS, is difficult to detect in the presence of moderate/marked background enhancement. Background parenchymal enhancement is hormonally mediated and does not correlate with mammographic density. Scheduling the patient’s MRI study during the first half of her menstrual cycle and discontinuing exogenous hormone therapy for several months before the study decrease the risk of excessive background enhancement. However, this is often not possible in patients with newly diagnosed malignancy, who need to avoid treatment delays.
Pectoral Muscle and Chest Wall Involvement
Tumors located posteriorly in the breast may invade the pectoral muscles and/or chest wall. Chest wall invasion by breast cancer is defined as tumor infiltrating the ribs, intercostal muscles, and/or serratus anterior muscle ( Figure 9.5 ). Tumors with chest wall invasion are classified as T4a, regardless of size (see Table 9.1 ). This results in a minimum TNM classification of at least stage IIIb disease (see Table 9.4 ), which correlates with a worse prognosis compared with the prognosis for patients who have tumors of comparable size without chest wall invasion. Preoperative treatment with chemotherapy and/or chest wall radiation, followed by more extensive surgery, including chest wall resection, is typically performed. Alternatively, a tumor that invades only the pectoralis muscle does not change the disease stage; however, it alters the surgical treatment. When a tumor superficially invades the pectoralis muscle, a portion of the muscle may be resected, or when deep muscle invasion is present, a radical mastectomy with removal of the entire muscle may be required ( Figures 9.6 and 9.7 ).

| Primary Tumor (T) | |
|---|---|
| The T classification of the primary tumor is the same regardless of whether it is based on clinical or pathologic criteria, or both. Size should be measured to the nearest millimeter. If the tumor size is slightly less or greater than a cutoff for a given T classification, it is recommended that the size be rounded to the millimeter reading that is closest to the cutoff. For example, a reported size of 1.1 mm is reported as 1 mm, or a size of 2.01 cm is reported as 2.0 cm. Designation should be made with the subscript “c” or “p” modifier to indicate whether the T classification was determined by clinical (physical examination or radiologic) or pathologic measurements, respectively. In general, pathologic determination should take precedence over clinical determination of T size. | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| Tis (DCIS) | Ductal carcinoma in situ |
| Tis (LCIS) | Lobular carcinoma in situ |
| Tis (Paget’s) | Paget’s disease of the nipple NOT associated with invasive carcinoma and/or carcinoma in situ (DCIS and/or LCIS) in the underlying breast parenchyma. Carcinomas in the breast parenchyma associated with Paget’s disease are categorized based on the size and characteristics of the parenchymal disease, although the presence of Paget’s disease should still be noted |
| T1 | Tumor size ≤ 20 mm in greatest dimension |
| T1mi | Tumor ≤ 1 mm in greatest dimension |
| T1a | Tumor > 1 mm but ≤ 5 mm in greatest dimension |
| T1b | Tumor > 5 mm but ≤ 10 mm in greatest dimension |
| T1c | Tumor > 10 mm but ≤ 20 mm in greatest dimension |
| T2 | Tumor > 20 mm but ≤ 50 mm in greatest dimension |
| T3 | Tumor > 50 mm in greatest dimension |
| T4 | Tumor of any size with direct extension to the chest wall and/or to the skin (ulceration or skin nodules). |
| Note : Invasion of the dermis alone does not qualify as T4 | |
| T4a | Extension to the chest wall, not including only pectoralis muscle adherence/invasion |
| T4b | Ulceration and/or ipsilateral satellite nodules and/or edema (including peau d’orange) of the skin, which do not meet the criteria for inflammatory carcinoma |
| T4c | Both T4a and T4b |
| T4d | Inflammatory carcinoma (see “Rules for Classification”) |


Mammography and ultrasound are typically limited in making the diagnosis of muscle or chest wall invasion. Posterior tumors are often difficult to include in the mammographic image, particularly when they are affixed to the muscle. Acoustic shadowing caused by many tumors interferes with the sonographic visualization of the posterior border of the mass and underlying muscle. Even when tumors are palpable and clinically suspected to invade the underlying muscle, the ability to preoperatively determine the extent of invasion is limited. Breast MRI is superior in making this diagnosis. The pectoral muscle and chest wall are well visualized on breast MRI. It has been shown that contrast enhancement of the pectoral muscles or chest wall muscles is the only reliable finding to predict invasion. The enhancement may appear infiltrative with preservation of the muscular architecture or it may be masslike. It should be noted that muscle enhancement related to a recent biopsy can result in a false-positive MRI appearance. Loss of fat planes or close proximity of the tumor mass to the muscle are not reliable indicators of invasion. Also, vessels feeding the tumor, which course through the muscle, are commonly visualized and do not indicate invasion.
Skin Involvement
Breast cancers invading the skin are classified as T4b (see Table 9.1 ), also resulting in at least stage IIIb disease (see Table 9.4 ). Skin invasion is defined as skin ulceration or tumor nodules within the skin. Involvement of the dermis alone does not meet the criteria for skin invasion to increase the tumor stage. Inflammatory breast carcinoma is a separate entity in which peau d’orange changes involve greater than one third of the breast, resulting from tumor micrometastases within the dermal lymphatics (to be discussed later). However, peau d’orange changes that do not involve more than one third of the breast—which would meet the criteria for inflammatory breast carcinoma—are also classified as T4b. Locally advanced breast cancer is at times associated with direct skin invasion, which is typically contiguous with the underlying mass and localized to the skin overlying the mass ( Figure 9.8 ). Skin enhancement demonstrated on MRI with this pattern suggests skin involvement. Skin edema and skin thickening (>3mm) may also occur as the result of lymphatic obstruction with or without malignant involvement.

Nipple Involvement
Malignant involvement of the nipple related to an underlying malignancy is an important factor for treatment planning because resection of the nipple areola complex (NAC) is required, usually with a mastectomy. The identification of NAC involvement by MRI can be difficult because of the normal physiologic enhancement of the nipple. Both a lack of nipple enhancement and bilaterally symmetric nipple enhancement are normal, while unilateral nipple enhancement raises suspicion for nipple involvement ( Figure 9.9 ). Findings most suggestive of malignant nipple involvement include unilateral nipple enhancement, which is contiguous with the underlying index tumor ( Figures 9.10 and 9.11 ). Other patterns of unilateral nipple enhancement that may suggest malignant involvement include adjacent skin enhancement and diffuse nipple enhancement. Rim enhancement or linear/periductal enhancement within the nipple may be seen with DCIS ( Figures 9.12 and 9.13 ). Although the sensitivity of MRI to detect nipple involvement is imperfect, it is important to report both the size of the tumor and its distance from the nipple. Tumor size greater than 2 cm and tumor-to-nipple distance of less than 2 cm may be significant factors in predicting nipple involvement.





Skin and nipple retraction are often obvious on clinical exam. However, subtle changes may not be appreciated. Retraction occurs as the result of tumor desmoplasia, which is identified on MRI as fine linear bands extending from the tumor to the skin or nipple, resulting in retraction. This finding may be best appreciated on the non–fat-suppressed T1 sequence. Although this should be reported, without corresponding abnormal enhancement of the skin, nipple, or muscle, it neither implies invasion nor alters the clinical stage.
Patient Selection for MRI Evaluation of the Ipsilateral Breast
Determining which patients are most likely to benefit from preoperative breast MRI has been the focus of a number of studies. Several studies have suggested that breast MRI may be most useful in women with mammographically dense breasts (>50% density). This is intuitive, given the known decrease in mammographic sensitivity related to breast density suggesting a higher likelihood of occult disease in these patients ( Figures 9.14 and 9.15 ). However, there may still be benefit for MRI in patients with nondense breasts (<50% density) because additional studies have found an equal number of occult malignancies in patients with newly diagnosed breast cancer with nondense breasts. Therefore preoperative MRI should not be reserved only for those patients with high mammographic density.


MRI has high sensitivity in detecting and evaluating invasive breast cancer. Evidence suggests that preoperative breast MRI is particularly useful in patients with a new diagnosis of invasive lobular carcinoma. Although invasive lobular carcinomas can be elusive on mammography because of their tendency for an infiltrative growth pattern, they most commonly appear on MRI as an enhancing mass. Reported mammographic sensitivities for invasive lobular carcinoma range from 34% to 81%, with MRI sensitivity range of 93% to 96%. MRI has also been shown to assess tumor size with higher accuracy than mammography. Lower reexcision rates for positive margins have been demonstrated when MRI is used preoperatively in patients with invasive lobular carcinoma. This is attributed to both the improved sensitivity of MRI over mammography in detecting invasive cancers and the known decreased sensitivity of mammography in detecting invasive lobular carcinoma ( Figure 9.16 ).

Although early reports asserted that DCIS was not well detected on MRI, more recent studies have shown that MRI is, in fact, superior to mammography in detecting DCIS. DCIS most commonly presents on MRI as clumped nonmasslike enhancement in a segmental, linear, or regional distribution. DCIS lesions produce a variety of kinetic curve shapes, including plateau and persistent curves. Thus the diagnosis should be suspected based on morphology rather than enhancement kinetics. Kuhl and colleagues found that MRI allows prospective diagnosis of DCIS even when the disease is undetected by mammography. In their study, MRI detected more cases of DCIS, including low, intermediate, and high grade, than did mammography. Of note, the sensitivity of MRI was higher with higher nuclear-grade DCIS, whereas mammographic sensitivity decreased with higher-grade DCIS. MRI sensitivity for high-grade DCIS was 98% compared with mammography sensitivity at 52%. Overall, the sensitivity for diagnosing all types of DCIS on MRI was 92% compared with 56% for mammography. This suggests that preoperative MRI should be useful to evaluate the extent of disease in patients with newly diagnosed DCIS, particularly with high-grade disease, as well as for invasive cancers with an extensive intraductal component ( Figures 9.17 and 9.18 ).


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree