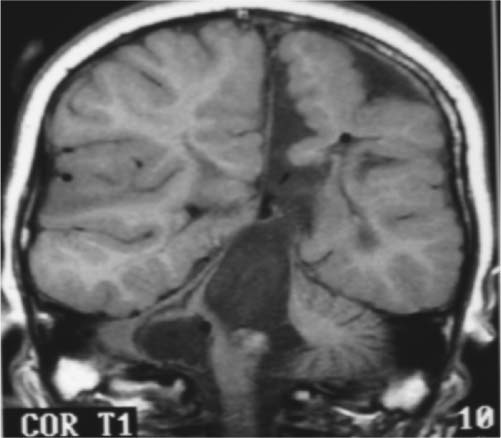
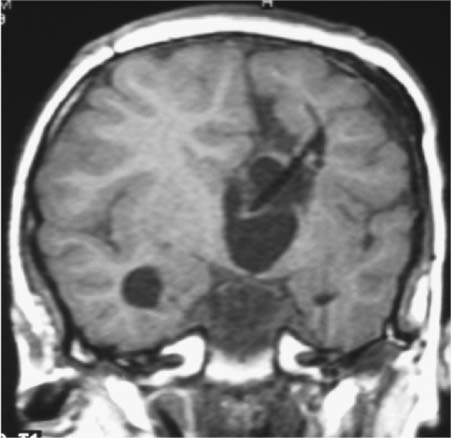
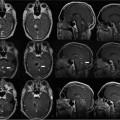
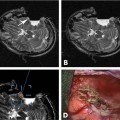
9 Intraoperative magnetic resonance imaging (iMRI) allows neurosurgeons to interactively perform surgery using MRI guidance. This relatively new technology provides exceptional visualization of intracranial anatomy and pathology. Its availability and use are quickly becoming a standard for comprehensive care hospitals and practices in the United States, Europe, and beyond. To embrace this technology, some of neurosurgery’s most difficult problems are being given renewed attention with an eye toward an iMRI solution. For example, traditionally one of the great challenges in neurosurgery has been to accurately cannulate the tiny ventricular system of a child with a shunt catheter. Likewise, deep cystic lesions next to critical parts of the brain in both the adult and pediatric populations are often equally difficult to cannulate and divert. In recent years, more refined techniques for placing these catheters have been evolving as the rate of technology development accelerates. Important milestones along the way have included the development of modern flexible plastic catheters, ultrasound/endoscopic assistance, and now iMRI guidance. Real-time or near-real-time image-guided catheter placement across the frontal, parietal, and occipital lobes has become an important new technique in complex cases. Another advantage is that, unlike other neuronavigation techniques such as computer-guided stereotaxy, iMRI allows the neurosurgeon to adjust for the brain shift that occurs once the cranium is open.1 With the advent of iMRI, catheter placement into dangerous locations such as the 4th ventricle or posterior fossa cysts near the brainstem have become a safer possibility. Alternate shunting techniques that have helped to lay the foundation for iMRI procedures include ultrasound- (US-) guided catheter placement in premature neonates, endoscopic third ventriculostomies, and image-guided shunting of complex multiloculated hydrocephalus or cystic lesions. In adults, the frontal horns are often large enough to allow blind cannulation of the lateral ventricle using anatomic landmarks only. The smaller the patient or lesion/ventricle though, the more difficult the target can be to access. The frontal approach is often preferable because it is familiar to most neurosurgeons. Likewise, in children the alternative occipital approach is a good second choice that takes advantage of common accessible landmarks and wide central access to the ventricular system. Both approaches are safe and in experienced hands can be performed accurately, but with varying skull geometries, ventricular shapes, and congenital anomalies, the approach can be very difficult. These customary methods could be improved upon so that placement of the catheter could be done in a single pass sparing the brain from repeated trauma. Clearly, the same concern extends to both adult and pediatric patients with complicated clinical scenarios. US techniques presaged the advent of modern MRI-guided practices. The first formal published description of US-assisted shunt catheter placement was by Shkolnik in 1981. In that paper, the author succinctly described the advantages of using image guidance. Most importantly, he described a new and reliable method of assuring that the final placement of the shunt tip was in an optimal site in the lateral ventricle.2 However, perhaps the greater gain with US was that the neurosurgeon could finely adjust the trajectory of the catheter in real time. Before then, there was no way to know with any immediate confidence that both the path through the brain and the final location were correct. In retrospect, US guidance seems crude compared with the detailed anatomy seen on MRI. Yet, in retrospect, the anatomic knowledge gained with US was leaps and bounds ahead of the blind passage technique.3–5 The ultrasound therefore laid the groundwork for iMRI in terms of guidance for catheter placement. If insertion of the catheter is undertaken in a patient with relatively normal anatomy, fine discrimination of cerebral structures may not be important. US would likely be a good choice and confer some speed and accessibility advantages for an experienced surgeon in that case.6,7 However, like other advances in technology, MRI and MR-guided surgeries improve on the prior ultrasound techniques with very fine detailed imagery which is especially important in cases with distorted, abnormal or small/pediatric anatomy. Similarly, with advances and miniaturization in optics, endoscopic techniques have also helped create the groundwork for MRI-guided surgery. In addition to the evolution of surgical techniques and instruments, there was a parallel improvement of diagnostic techniques. Both processes of development influenced each other. Modern diagnostic imaging is able to provide us with almost all the individual anatomic and pathologic or anatomic details of a specific patient and is able to show us the individual anatomic windows. With the knowledge of these details, it is possible to target and to treat an individual lesion through a relatively small (keyhole) approach.8,9 The problems of adopting a miniature incision are narrow viewing angles, reduction of light intensity in the operating field, and the necessity for almost coaxial control of the microinstruments. The rigid endoscope with its rounded wide view lens, powerful light source, and relatively small diameter made directed, exploratory microsurgery possible. Procedures at the skull base or deep in the brain such as a third ventriculostomy could be done with the aid of real-time feedback and continuous trajectory adjustment based on up to the moment data. iMRI developed from, and alongside these precursor techniques represents a natural next step in the attempt to embrace new technology, decrease morbidity, and to refine the accuracy of our surgeries. The first MRI-guided surgical suites came on-line in the mid-1990s; since then the technology has matured to much higher magnet strength and better designs of both MRI-compatible surgical instruments and the suites themselves. Tumor surgeons were the first to embrace this leap in technology. One group found that 41% of their glioma resections (grades 1–4) were extended after imaging, increasing the percentage of gross total resections from 27 to 40%.10 Next, deep brain stimulator surgery with its high demand for precision targeting of deep seated nuclei was adapted and augmented by the push toward iMRI. At the same time, cannulation of complex cystic lesions and difficult shunt placement procedures were being attempted. This was aided in part by the development of tools and scanning protocols designed to facilitate brain lesion needle biopsy. Early iMRI-guided brain biopsies were performed freehand in the same way that early computed tomography-(CT-) guided brain biopsies were accomplished. Initially, there was no way to direct the brain biopsy needle toward the target or to stabilize the needle after the region of interest had been reached. The need for needle stabilization devices soon became apparent and they were rapidly designed and developed. Early work on a disposable trajectory guide (Navigus, Medtronic Navigation, Inc., Louisville, CO) was undertaken at our facility. This was done in combination with a unique targeting technique known as prospective stereotaxy to perform brain biopsy in near-real time using a 1.5 T iMRI system.1 Certainly, if this new method could work for needle biopsy, it could just as easily help place a drainage catheter. At the University of Minnesota, we have performed over 1000 intraoperative MRI cases in the decade since coming on-line in 1996. The different neurosurgeons within the department found a wide variety of uses and applications for MRI-guidance. Deep brain stimulator lead placement, tumor removal/biopsy, and catheter placement in anatomically difficult patients are some of the typical indications. There are in fact two different surgical MRI suites at two different hospitals in our academic program in Minneapolis. One is a 1.5 tesla system and the second has a 3.0 tesla magnet. The higher resolution or speed in acquisition can be helpful in working close to vital and sensitive structures such as the medulla oblongata where millimeters of accuracy count. The drive to use iMRI in pediatric and adult oncology cases has been strong. More often, it is the pediatric cases that present the kind of technical challenge where interventional MRI can be the greatest help. In an early paper covering cases from 1997 to 2000, nine posterior fossa intraoperative magnet cases out of 11 were pediatric. The mean age was 6.4 years and the median age 7. Seven mid-line craniotomies were performed, of which three were reoperations. Two were burr hole placements, one for cyst aspiration with P32 instillation, and the other for tumor biopsy.11,12 In each case, iMRI was chosen as the superior option for the complexity of the case in the setting of such diminutive anatomy. Nimsky et al indicate that among the iMRI pediatric cases at their institution, it was the monitoring of catheter placement and consecutive cyst alterations that proved the most beneficial application of the technology. In many of their catheter placement cases, intraoperative imaging resulted in a modification of the surgical strategy. It served as an intra-operative quality control that helped them to monitor the effects of the ongoing surgery (e.g., the extent of a resection or depth of a catheter), in comparison with the treatment plan. They state that besides its application in brain tumors, iMRI has also proven to be particularly helpful in children undergoing complicated catheter placements for cyst drainage, as well as in pituitary and epilepsy surgery.13 An example of how to best use the iMRI for catheter placement is a case where there was an expanding cystic lesion adjacent to a sensitive, vital structure. A 4-year-old child with cerebral palsy was brought in to the emergency department with recurrent and worsening hydrocephalus despite an existing traditional frontal ventriculoperitoneal shunt. From birth, the boy had had a complex cystic lesion that was incorporated into his ventricular system. Recently, the lesion had begun expanding the 4th ventricle (Fig. 9.1). This had worsened over a period of months and with it a gradual neurologic decline. iMRI was used on two different occasions in attempts to place a catheter that adequately controlled and drained the expanding cystic lesion. First, a left-sided frontal approach was used to place a 3rd ventricular/cyst catheter in the hope that the cysts and ventricular system were in continuity and the entire system could be decompressed from above (Fig. 9.2). Fig. 9.1 Presenting lesion in a 4-year-old boy with cerebral palsy and frontal ventriculoperitoneal shunt failure now with an expanding 4th ventricular cyst lesion. Fig. 9.2 Initial intraoperative magnetic resonance imaging shunt placement, but 4th ventricular cystic lesion continued to expand. Follow-up CT scans showed this was only partially successful and a more definitive procedure would have to be attempted from below to fully decompress the 4th ventricle. The patient returned to the operating room a few days later. This time a suboccipital approach was undertaken and the catheter was meticulously placed within the 4th ventricle cystic lesion along the plane of the brainstem with good results (Figs. 9.3 and 9.4). Catheter placement required two passes because the first trajectory caused the catheter tip to lie against the anterior wall of the ventricle. This was seen immediately and corrected with a subsequent pass using more frequent updates of MRI scanning as the catheter was slowly advanced into better position. This would likely not have been recognized intraoperatively using other imaging techniques and the implications of not correcting it immediately would have been critical. Any catheter migration or even irritation of the brainstem could have been devastating. Such quality control and precise localization is a truly unique advantage of iMRI-guided procedures.
MRI-Guided Catheter Placement
Milestones Leading to iMRI Shunting Techniques
Clinical Experience
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree