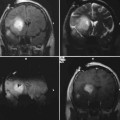
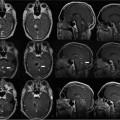
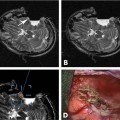
24 Magnetic resonance imaging-guided focused ultrasound (MRgFUS) is expected to revolutionize disease treatment including, but not limited to, the ablation of benign and malignant tumors and vascular malformations as well as the nonablative targeted delivery of therapeutic drugs, genes, and antibodies. Coupling magnetic resonance imaging (MRI) guidance with advanced phased-array ultrasound (US) technology, coined MRgFUS, has allowed FUS therapy to emerge as a viable noninvasive alternative to invasive and minimally invasive treatment. In 1880 Pierre and Jacques Curie described the basic tenet on which modern US is founded, the piezoelectric effect (the production of acoustic energy when electric current is applied to a piezoelectric crystal).1 However, it was not until 40 years later, in 1918, with the development of technologic advances in electronics, that US, as we know it today, was created. This era of modern US began when Paul Langevin, a physicist, designed the sonar for submarine detection by interposing a mosaic of thin quartz crystals glued between two steel plates.2,3 In 1938, Raimar Pohlman noted the first “therapeutic effect” of US on human tissue using low-intensity US to treat patients with inflammatory conditions.4 Langevin subsequently went on to make the first observations of high-intensity focused ultrasound (HIFU) by demonstrating alterations in the swimming patterns of fishes during the emission of sound waves.2 The first demonstrated use of HIFU on biologic tissue occurred when Lynn and Putman treated 37 animals by delivering targeted FUS to specific areas of the brain.5–7 Fry then accentuated the potential of FUS as an alternative to open neurosurgery8–10 following the demonstration of successful pinpoint ablations through a craniotomy window in primates. Even though their clinical results were mixed, Fry and Heimburger subsequently went on to establish the safety of HIFU to destroy brain tumors after craniectomy.11,12 Additionally, in 1955 Barnard et al led the first study of US-induced disruption of the blood-brain barrier (BBB).13 Similar research was subsequently performed by the Ballantine and Lele team,14 which described the biologic effects of FUS on sonicated brain tissue.15,16 Inspired by the results of previous research and clinical trials, Petter Lindstrom, considered by many as one of the pioneers of “bloodless surgery,” used HIFU lesioning to treat patients with psychiatric disorders, epilepsy, and intractable pain from carcinomatosis.17 Introduced to this functional neurosurgical method by Lindstrom, Leksell, the acknowledged pioneer of radiosurgery, was the first to develop a special adapted frame for an US transducer for the purpose of HIFU lesioning. For Leksell, the major limitation precluding the use of HIFU as a therapeutic device included the (1) need for a craniotomy, (2) lack of reliable imaging tool to plan treatment, and (3) lack of real-time intraoperative feedback and assessment of the targeted lesions. He subsequently abandoned HIFU as a lesioning device and focused on ionizing radiation, an effort that resulted in the development of the gamma knife in 1967.18 The above-mentioned limitations have been successfully addressed with the development of magnetic resonance imaging (MRI) by Lauterbach and colleagues in the 1980s. This was a significant contribution to the advancement of FUS as an image-guided therapeutic instrument. MRI is a proven reliable and sensitive imaging modality used to define the target (i.e., tumors) and to plan treatment preoperatively. Coupled with the development of MRI thermometry19–23 in the early 1990s, MRI can provide real-time intraprocedural feedback, permitting a controlled treatment environment. MRI thermometry detects temperature changes with marked accuracy and sensitivity based on shifts of proton resonance frequency.24 Subsequent research found that temperature elevations correlated well with the degree of tissue damage,25,26 making MRI thermal imaging the modality of choice for intraprocedural evaluation of treatment performance. At the same time, the development of phased-array transducers27–29 led to the potential of transcranial delivery of therapeutic US through an intact skull, precluding the need for craniotomy. Alongside MRI, FUS makes a strong case as a safe, noninvasive, and practical alternative to conventional invasive treatment options. MRgFUS combines and integrates two modalities into a single image-guided therapy delivery system. FUS is the therapeutic instrument causing direct US-induced changes in the targeted tissue that include nonthermal (nonablative) and thermal (ablative) tissue changes. As an ablative tool, FUS ablates deep tissue volumes without disturbing the overlying tissue (near-field tissue).30,31 Coupled with the latter, MRI provides both guidance and immediate postprocedural evaluation of the treatment. It is used preoperatively to accurately identify the target tissue, intraoperatively to monitor treatment in real-time via MR thermometry and postoperatively to validate treatment success. As nonablative therapy, FUS can reversibly disrupt the BBB, resulting in an increase in cerebrovascular capillary permeability and, therefore, an increase of the flux of relatively larger, non-lipophilic, and high-molecular-weight molecules that would otherwise not cross the BBB.32,33 MRgFUS offers clear, unambiguous advantages over other current treatment modalities. It is noninvasive and does not deliver ionizing radiation to either patient or physician. In contrast to the gamma knife, which lends itself to only a single treatment session (irrelevant of the treatment success) due to its toxic cumulative effects, FUS allows for unlimited treatments in a single session as well as unlimited repeated sessions over time. Unlike in both surgery and radiotherapy, real-time noninvasive intraprocedural monitoring can be performed with remarkable accuracy using MR thermometry, which gives outstanding anatomic definition of the target site. MRI thermal imaging is the sole modality available to evaluate thermal tissue effects; it is used to determine the therapeutic endpoint in FUS ablation, and the success of treatment.34,35 Secondary neoplasms, caused by radiosurgery and radiotherapy, are not seen with FUS therapy. Furthermore, the thermal gradients of FUS are much narrower than the dose-curves in radiosurgery; therefore, they are more precise and less thermally damaging to the adjacent tissues. However, both technical and inherent limitations remain, including lengthy treatment sessions largely due to small focal volumes of ablation per sonication and to relatively long cooling times that prevent the accumulation of heat beyond the focal volumes. Ongoing research is striving to annihilate or at least decrease to a certain extent these limitations. Long treatment times reflect the small volume of ablation per sonication, and the cooling time and MRI thermometry acquisition needed between each sonication. However, increased volumes can be obtained by creating multiple focal points as well as overlapping sonications. Due to the absence of an adequate acoustic window for the transmission of sound waves, as opposed to the creation of an acoustic window through a craniotomy site, the intact osseous skull creates two major challenges when performing transcranial FUS: (1) heating caused by the absorptive properties of bone, and (2) the inability to establish a focal point of convergence due to scatter, resulting in inaccurate targeting and uncontrolled energy deposition. Current phased-array transducers address these challenges by allowing for transcranial focusing28,36 without overheating the skull by using models derived from preprocedural computed tomography (CT) scans used to compute and correct for skull thickness and density.27 Organ motion during treatment is important as small motion can cause large shifts in focal points, resulting in an imprecise and incorrect zone of ablation. For the latter, intraprocedural MRI and adaptation of the phased-array transducer elements are enjoined to retarget and refocus, yielding for a controlled, precise deposition of heat to the intended target lesion. Organ motion also prevents correct temperature measurements with phase difference-based thermometry. For the treatment of moving organs like liver and kidney, more research is necessary to achieve the same precise treatment that is possible in the brain. By addressing the above-mentioned limitations, the procedure will decrease in complexity, require less manpower, and translate into an increased acceptance of clinical applications by clinicians. The thermal effects of FUS on tissue depend on the acoustic intensity and the absorption coefficient of the tissue. The ablative effects on tissue are caused by thermal heating that is predominantly a product of the absorption coefficient of the tissues. Given the dependency on the acoustic intensity of the US beam, generation of thermal energy is mostly on the focal point of convergence where the intensity is highest. Using temperatures higher than 56 to 60°C, focused sonication with only a few seconds duration causes thermal coagulation.29 This nonselective tissue coagulation necrosis results in the killing of both neoplastic and nonneoplastic cells; it is an unambiguous heat-induced cell death occurring secondary to protein denaturation that beckons the need for a reliable and sensitive imaging method for monitoring tissue changes. Given that it can accurately detect fluctuations of less than 2 to 3°C, MR temperature-sensitive imaging (MR thermometry) fits these criteria.34,35 The all-or-none ablative effect of FUS irrespective of tissue type resembles surgical treatment. However, unlike surgery, the nonablated region is preserved. In contrast, hyperthermia, which also has tissue ablative effects, delivers relatively lower temperatures (43°C) over a longer period of time (30–60 minutes),37,38 thereby causing more selective cell damage. Percutaneous heat-conducting probes cause a shallow temperature gradient due to the steep heat sink effects from perfusion and blood flow that dissipate heat farther from the single source probe. These probes, therefore, need long exposures to increase the ablative volume. Additionally, unlike FUS, the probe ablation zone cannot be tailored to fit the geometry of the target volume. Regardless of the ablative source, therefore, to monitor therapy and avoid under- and overtreatment, spatial and temporal monitoring of thermal heating is of utmost importance. The nonthermal, nonablative effects of FUS on soft tissue are due to multiple complex mechanisms, the most important mechanism being cavitation that results in the following: (1) acoustic streaming, (2) mechanical (acoustic radiation) forces, and (3) inertial (transient) cavitation.39–42 Inertial cavitation is likely responsible for most of the nonthermal biologic effects. These mechanisms result from the interaction of microbubbles with US.43,44 Microbubbles can be induced by the high-intensity US itself and generated within the native sonicated tissue, or preformed microbubbles (clinically used US imaging contrast agents) can be intravenously administered, the latter termed microbubble-enhanced therapeutic ultrasound.45–48 At low acoustic power, oscillating microbubbles contribute to disruption of the BBB by producing shear stresses on the cells by microstreaming fluid around the bubble.43,44 Mechanical radiation forces cause the bubble to move in the direction of the wave propagation, exerting force on the endothelium perpendicular to the direction of the blood flow and length of the vessel.49 This action causes localized stretching of the cell membrane and stimulation of the endothelial cells, inducing its biologic effects on the BBB. At high acoustic pressures, FUS causes oscillation and the rapid growth of bubbles, that on implosion (violent collapse; also known as inertial cavitation), unpredictably release stored energy in the form of shock waves.33,43,44 It also produces high velocity jets50 and free radicals.51 These biologic effects disrupt the cell membrane and endothelial tight junctions, allowing uncontrolled deposition of thermal heat. Due to its unpredictable, nonlinear effects on heating that can lead to hemorrhage and unwanted tissue destruction,52 cavitation is an undesirable effect of thermal ablation where precise, controlled targeted lesion ablation is needed. In nonthermal ablations, however, the effects of cavitation can be exploited. For example, disrupting the BBB, at lower energy levels can increase cell and vascular permeability. With the development of contrast microbubble agents,42,45,53 disruption of the BBB can be performed at even lower acoustic pressure levels, decreasing the above-mentioned side effects of uncontrolled heating including hemorrhage and unwanted tissue destruction.40 Preprocedural planning is critical to evaluate and precisely define the anatomy of the desired target lesion and adjacent structures, determine the procedural approach, and plan a viable trajectory for the delivery of focused sonications. Given the ability to visualize lesions, MRI and CT scanning are pivotal in planning, optimizing, and controlling treatment. With its superior signal-to-noise ratio (SNR) and ability to provide a three-dimensional spatial map of the targeted tissue volume, MRI remains the imaging method of choice for surgical and procedural planning including the defining of correct anatomic (and functional) localization and mapping, spatial volume, size and extent of the targeted lesion, the margins of the lesion itself and surrounding critical anatomic structures, as well as providing exquisite tissue characterization. MRI’s accuracy and reliability far outperform other imaging modalities. Furthermore, by increasing SNR that results in superior resolution, higher field strengths provided by 3.0 T confer added sensitivity over 1.5 T MRI systems with consequent improvement in tumor margin delineation. However, because of the inherent infiltrative nature of most malignant tumors and the limited sensitivity of MRI to detect microscopic infiltration of tumor cells into normal-appearing tissue, precise definition of the margins of malignant tumors remains a challenge and incomplete treatment can result. Similar in importance to MRI for preprocedural planning, the CT scan of the head is pivotal for planning transcranial MRgFUS therapy. Given its high acoustic attenuation coefficient, bone represents a relative barrier that is not impenetrable to the transmission of US sound waves and acoustic energy deposition,54 unlike air- and gas-containing cavities that represent absolute barriers to acoustic energy deposition. The high acoustic impedance of the osseous skull is proportional to skull thickness and density. It is evaluated and computed from the information obtained by the CT scan.27 The acoustic impedance is a result of and due to multiple factors including but not limited to scatter and absorption of most of the energy.55 Absorption of energy by bone also contributes to the heating of the skull, an important limitation in transcranial FUS. Heating of the skull due to energy absorption and severe aberration and phase distortion due to scatter is corrected by current phased-array transducers via acoustic modeling.27
MRI-Guided Focused Ultrasound Surgery in the Brain
MRgFUS: Introduction and History
MRgFUS: General Concepts, Advantages, and Limitations
MRgFUS: Mechanism of Action
Thermal (Ablation) Effects
Nonthermal (Nonablation) Effects
MRgFUS: Approach
Preprocedure Stage: Planning and Localization
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree