6 MRI-guided Interventions After mammography and ultrasound (US), magnetic resonance imaging (MRI) of the breast is the third diagnostic imaging technique used in the diagnostic work-up of breast findings. In contrast to the other techniques, MRI primarily gives information on the vascularization of intramammary structures. Because malignant tumors typically have an increased vascularization attributed to tumor neoangiogenesis, this phenomena can be visualized in the slice images acquired after administration of a contrast medium to differentiate between benign and malignant findings of the breast. Numerous studies substantiate that magnetic resonance (MR) mammography is clearly superior to other imaging modalities in the detection of invasive breast cancers. Furthermore, first studies show that MRI using HR methods is also superior in the detection of intraductal tumors. Currently, MRI of the breast has a sensitivity of approx. 85–95%. The specificity achieved by working groups performing MR mammography with a high technical and procedural quality is approx. 90%. In general, the performance of contrast-enhanced MR mammography is expedient when less costly examination techniques such as mammography and US are of limited diagnostic value. This is primarily the case when breast tissue is mammographically dense (American College of Radiology [ACR] density types III and IV) and inhomogeneous on US. Several studies have shown, however, that even when breast tissue is not dense, mammographically occult carcinomas can be detected by MR Mammography. Generally accepted indications for the performance of MR mammography are preoperative local staging of patients with a lesion highly suspicious of malignancy (BI-RADS 5; Breast Imaging Reporting and Data System—classification system according to the ACR), or with a proven malignancy after percutaneous biopsy (BI-RADS 6). The relevant questions to be answered in this constellation refer to the tumor size; the presence of an extensive intraductal component (EIC); a multifocal, multicentric, and/or contralateral tumor. In addition, MRI provides important criteria for the differentiation between scar tissue and a malignant tumor. Numerous studies have also documented the great value of MR mammography for women with an increased risk of developing breast cancer (e. g., BRCA [breast cancer] gene mutation carriers). In fact, MRI of the breast is now considered the most important examination technique for these women because it has been found to be superior to all other imaging modalities in detecting breast cancer. Other reasonable indications for performing MR mammography include the monitoring of patients undergoing neoadjuvant chemotherapy, and the search for a primary tumor in cases of CUP syndrome (cancer of unknown primary). Ultimately, MR mammography is regularly used as a problem solver when an ambiguous finding cannot be resolved by mammography and a breast US examination. Apart from these indications relating to tumor detection and visualization, an MRI examination without the administration of contrast material is the most reliable examination technique for the diagnostic work-up of prosthesis complications. A modern strategy in breast diagnostics is the “Göttinger Opti-pack.” It is the combination of a bilateral digital mammogram in mediolateral oblique (MLO) projection with a contrast-enhanced MR mammography. This combination has the highest sensitivity for the detection of breast cancer at the lowest possible radiation exposure. It is (if one does not take the cost into account) the ideal strategy in the early diagnosis of breast cancer. Equipment. MR mammography is preferably performed in a whole-body magnet with a field strength of 1.5 T. Systems with lower field strengths no longer satisfy the quality requirements for a high-resolution breast examination. Positive reports have also been published pertaining to the use of 3.0-T magnets. A breast MRI examination is performed using a dedicated, 4- or 8-channel phased array surface coil made to fit the breast form. To perform a breast intervention, an open coil that allows access to the breast from the lateral and/or medial aspect, or from the cranial aspect, is required. In addition, open breast coils allow good immobilization of the breast by using integrated compression devices during a diagnostic MR mammography examination. Contrast material. MRI measurements are performed using T1-weighted gradient echo (GE) sequences, which have a great sensitivity for paramagnetic contrast materials. Measurements are typically performed before and repeatedly after intravenous (IV) administration of an appropriate MRI contrast material in the cubital vein. The amount of contrast material required (e. g., gadopentetate dimeglumine) is 0.1 mmol/kg body weight (BW). In addition to the T1-weighted sequences, water-sensitive sequences (e. g., T2-weighted, turbo spin echo [TSE], T2-weighted inversion recovery [IR]) should be performed with identical slice positioning before performing the contrast-enhanced dynamic measurements. Minimum and preferred requirements. To attain a high-quality, contrast-enhanced MR mammography, standardized methods and techniques must be employed. Table 6.1 lists the minimal requirements and the currently preferred parameters used for performing a high-standard MR mammography.
MR Mammography
Significance
Indications
Technique and Methodology
Criteria | Minimum requirement | Optimal settings |
Technique | 2D/3D | 2D/3D |
Spatial resolution: slice thickness | ≤ 4 mm/slice | ≤ 3 mm/slice |
Spatial resolution: matrix | 256 × 256 | 512 × 512 |
Temporal resolution | < 2.5 minutes/sequence | < 2 minutes/sequence |
TE | In phase | In phase |
FOV | 350 | 350 |
* Recommended by the “AG Mammadiagnostik” of the German Radiology Association, 2003.
Abbreviations: TE = echo time; FOV = field of view.
Time of examination performance. In premenopausal women, MR mammography should be performed in the second week of the menstrual cycle (less optimally in the third week). The first and fourth week of the menstrual cycle should be avoided. In certain cases (e. g., local pretherapeutic MRI-staging of women with a lesion in the BI-RADS category 6), however, a prompt examination without consideration of the menstrual cycle is indicated. The interpretation of MR mammography examinations performed on postmenopausal women should take hormone replacement therapy into account.
Terminology
Essentially, three types of contrast enhancement are differentiated in the analysis of MR mammography findings (Fig. 6.1).
Foci. Foci are spotty or blotchy enhancing breast areas up to 5 mm in diameter. A bilateral, symmetric, focal enhancement pattern within the parenchyma is often a physiologic finding, representing enhancement within the breast lobuli. It is usually a harmless and normal finding, and therefore does not necessitate short-term follow-up. However, a single focus or multiple foci seen near a malignant lesion have a higher probability of representing a carcinoma or satellite lesions.
Mass lesions. Mass lesions are space-occupying, three-dimensional (3D) findings with increased vascularization and a diameter over 5 mm. Interpretation of such lesions takes multiple characteristics into account, including morphologic and hemodynamic criteria (see Göttingen Score).
Nonmass lesions. Nonmass lesions of the breast are characterized by a diffuse uptake of contrast material, which typically respects lipomatous structures. The differential diagnosis includes:
 an inflammatory process
an inflammatory process
 a radial scar
a radial scar
 a malignant process (ductal carcinoma in situ [DCIS], invasive lobular carcinoma)
a malignant process (ductal carcinoma in situ [DCIS], invasive lobular carcinoma)
 normal findings
normal findings
Evaluation Criteria
Evaluation criteria are essentially divided into morphologic and dynamic criteria. With the increasing spatial resolution of modern MR equipment, however, morphologic criteria are attaining greater importance (Table 6.2). The dynamics of contrast enhancement is differentiated into a so-called early or initial, and late phase or postinitial.
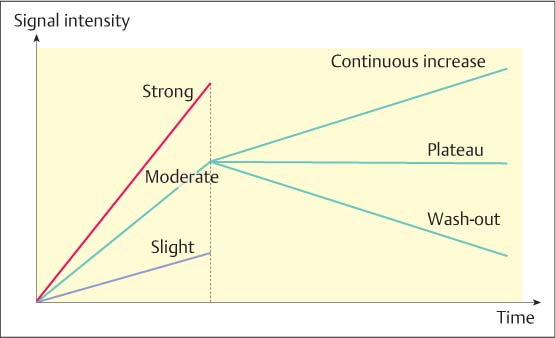
Initial phase. The first 3-minute period after contrast administration constitutes the initial dynamic phase. The so-called initial signal increase (Sinitial) is calculated as the percentage of signal increase (maximum signal Smax within the first 3 minutes after contrast administration) in relation to the initial signal in the pre-contrast image (Sprecontr). Typically, the initial signal increase is classified as slight, moderate, or strong. The quantitative assignment of the percentage signal increase to one of these classes is dependent upon the examination technique and methodology (e. g., contrast material dose, field strength, 2D- or 3D-measurement technique) and must therefore be adjusted based on the user.
Sinitial =(Smax − Sprecontr)/Sprecontr × 100 [%]
Late phase. The late or postinitial phase constitutes the period between the third minute after contrast administration and the end of the dynamic measurements, typically 8 minutes after contrast administration. The postinitial signal behavior takes the maximum signal within the first 3 minutes and the final signal (Sfinal) after 8 minutes into account. If the signal intensity value between these measurements increases (> 10%), then the postinitial signal behavior is described as a continuous increase. If the signal intensity value between these measurements decreases (> 10%), then the postinitial signal behavior is described as having a wash-out effect. When the signal intensity values remain relatively constant (< 10% difference), then this is considered to be a postinitial plateau (Fig. 6.2).
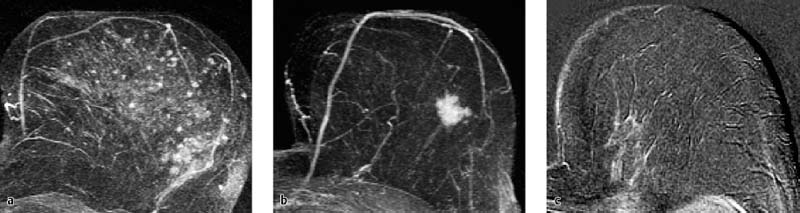
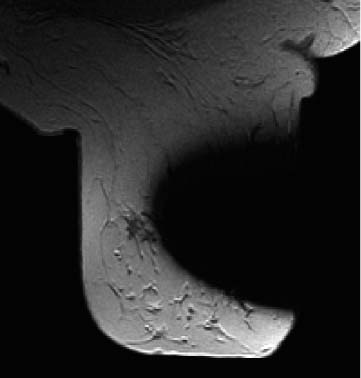
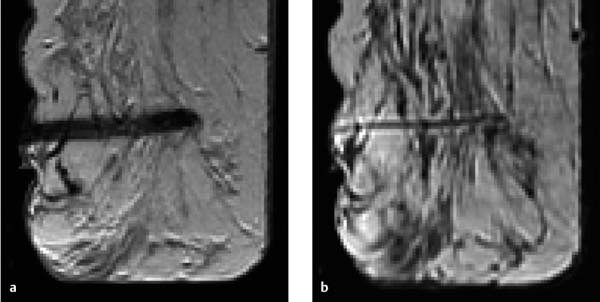
Fig. 6.1a–c Various types of contrast-enhancement in MR mammography.
a Foci (adenosis).
b Mass lesion (IDC).
c Nonmass lesion (ILC).
Spostinitial =(Sfinal − Smax)/Smax × 100 [%]
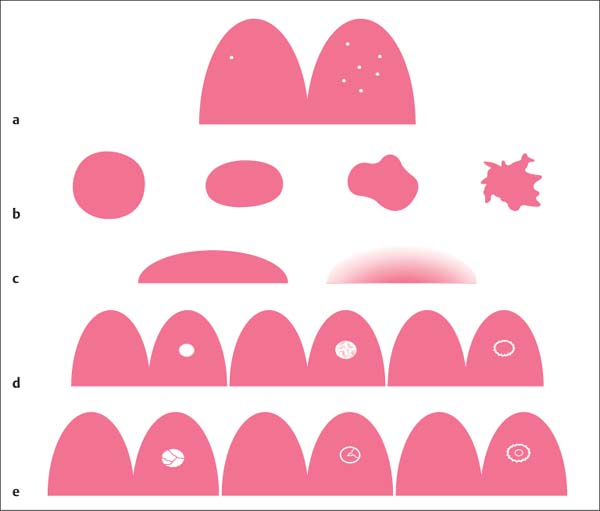
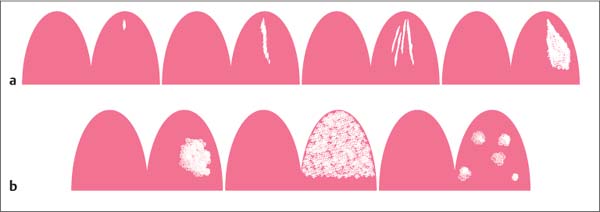
Morphologic criteria. Morphologic criteria are especially applied to the evaluation of mass lesions. The most important criteria include shape, margins, and internal enhancing pattern (Table6.2, Fig. 6.3). Nonmass lesions, however, also display different morphologic characteristics which should be used for their description (Fig. 6.4).
Fig. 6.2 Diagnostic criteria in MR mammography kinetic curves. Dynamic criteria in the initial and postinitial phases.
No single feature alone, however, can determine the assessment of an ambiguous finding. In fact, the application of multiple diagnostic criteria in the evaluation of MR mammography findings has been shown to significantly increase the specificity of this method. The Göttingen Score (Tables 6.3 and 6.4), for example, is a scoring method developed to facilitate the assessment of MR mammography mass lesions. By evaluating several diagnostic criteria, a lesion can be assigned a point score, which can then be translated to a MR mammography BI-RADS category (Tables 6.4 and 6.5).
Furthermore, a description of the internal enhancement pattern can also be applied to help assess a lesion. These include nonenhancing internal septations, enhancing internal septations, and a central enhancement (Fig. 6.3e). Nonenhancing internal septations (dark septations) are typical for myxoid fibroadenomas and represent non- or slightly enhancing fibrotic strands within the tumor, i. e., an additional criterion for benignity. Enhancing internal septations and central enhancement within a ring enhancement are indicative of proliferative changes within the tumor. They are more likely a sign of malignancy.
Criteria | Description |
Shape | Round, oval (more likely benign) Irregular (more likely malignant) |
Margins | Well circumscribed (more likely benign) Indistinct (more likely malignant) |
Internal contrast enhancement pattern | Homogeneous (more likely benign) Inhomogeneous (unspecific) Ring enhancement (more likely malignant) |
Fig. 6.3a–e Foci and mass lesions.
a Focus/foci (up to 5-mm diameter).
b Mass lesion—shape: round, oval, lobulated, irregular (from left to right).
c Mass lesion—margins: well circumscribed (left), indistinct (right).
d Mass lesion—enhancement pattern: homogeneous, inhomogeneous, ring enhancement (from left to right).
e Mass lesion—internal septations: nonenhancing septations, enhancing septations, central enhancement (from left to right).
Fig. 6.4a, b Nonmass lesions. a Focal, linear, dendritic, segmental enhancement (from left to right). b Regional, diffuse, multiple enhancements (from left to right).
Total points | MR mammography BI-RADS category |
0–1 | MR mammography BI-RADS 1 |
2 | MR mammography BI-RADS 2 |
3 | MR mammography BI-RADS 3 |
4–5 | MR mammography BI-RADS 4 |
6–8 | MR mammography BI-RADS 5 |
Particularities of MRI-guided Interventions
Materials. MRI-guided interventions must accommodate the special conditions encountered during an MRI scan. This pertains especially to the extremely strong magnetic field, which prohibits the use of customary ferromagnetic materials (Fig. 6.5). Biopsy and localization grids, post and pillar systems, biopsy equipment, as well as all materials inserted into the breast (coils, clips, wires) must be made of MRI-compatible materials, usually plastics or titanium- and nickel-based alloys (Fig. 6.6). These alloys cause only slight susceptibility artifacts and can therefore be distinctly seen. On the other hand, they do not cause a significant interference during imaging, and therefore do not mask relevant findings and fine structures.
The degree of signal extinction caused by susceptibility artifacts is dependent upon the composition of the materials used, and upon the examination parameters (among other factors). In comparison to GE sequences, for example, T1-weighted SE sequences have a lower response to contrast materials and require a longer examination time. They show significantly less interfering signal alterations and are therefore well suited for the final documentation of localization materials in the breast.
Localization fixtures must also be made of MRI-compatible materials without ferromagnetic substances, which cause interference in the acquired images. Plexiglas materials have proven suitable for this purpose because they can be adequately processed and can withstand a large amount of material stress. In addition, all utensils must meet the appropriate hygienic requirements.
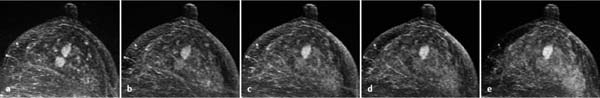
Diagnostic time slot. Another special condition encountered during MRI-guided interventions concerns the detection of ambiguous breast findings: the depiction of hypervascularized findings after IV contrast administration is usually only possible within a time interval of a few minutes. The target finding must be clearly identified within this time slot (Fig. 6.7). All the following steps of an intervention are performed later, when the target lesion can generally no longer be differentiated from the surrounding, now also enhancing healthy breast tissue. If the target lesion cannot be identified within the diagnostic time slot, or a relevant displacement of breast tissue takes place in the course of the intervention procedure, then the intervention must be discontinued. Generally, the repeat intervention procedure can be performed on the following day at the earliest.
Fig. 6.5 Unacceptable susceptibility artifact. Strong signal extinction due to use of inappropriate ferro-magnetic needle in MRI intervention.
Fig. 6.6a, b Acceptable and optimal susceptibility.
a Needle shows acceptable signal extinction in GE sequence image.
b Very slight susceptibility artifact and more precise depiction of the same needle in a spin-echo (SE) sequence image.
Fig. 6.7a–e Diagnostic time slot during MR mammography. Different perfusion patterns of two adjacent mass lesions: dorsal mass = carcinoma, ventral mass = fibroadenoma. Early enhancement of both lesions (a). Wash-out in the carcinoma during the second and third measurement (b–c) (limited time frame for visualization = approx. 3 minutes). Increasing enhancement within the fibroadenoma during entire examination sequence (a–e).
MRI-guided Vacuum-assisted Biopsy: Diagnostic Procedure
Indications and Objective
The main indication for the performance of MRI-guided biopsy is the diagnostic work-up of a suspicious finding on MR mammography (MR mammography BI-RADS category 4 or 5) that has no clearly correlating clinical or other image modality finding. Occasionally, an MRI-guided biopsy can be performed for the diagnostic work-up of a finding in the category MR mammography-BIRADS 3. This is indicated when the patient wishes a definitive diagnosis, or to rule out multicentricity and/or bilateral breast cancer if the patient has a BI-RADS 5 finding at another location.
The purpose of an MRI-guided percutaneous biopsy is to attain a definitive histopathologic diagnosis of ambiguous MRI findings by obtaining representative tissue samples. Thus, it has a diagnostic, and not a therapeutic objective, and does not normally aim to remove the ambiguous finding completely. Small intraductal papillomas, however, are the exception. Here the complete removal by VAB with a therapeutic objective can be a reasonable alternative to surgery. The procedural strategy in this context requires that the harvested samples be systematically harvested and arranged for pathologic study in a structured manner.
Patient Positioning
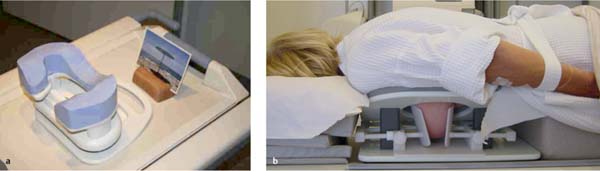
MRI-guided interventions are performed with the patient lying in the prone position and the breast hanging freely inside the surface coil. If the target is accessed from the lateral aspect of the breast, then the opposite breast can also hang inside the surface coil. If the target is accessed from the medial aspect of the breast, then the opposite breast must be positioned outside the surface coil. The patient’s head may be positioned to one side, or preferably face down because this usually causes less cervical complaints. Earplugs or music headphones can increase patient comfort in this situation. In the face-down position, it is also possible to create a free view by means of an integrated mirror optic (Fig. 6.8a). Based on the coil design, the arms may be positioned above the head or alongside the body. When positioning the arms alongside the body, use a bathrobe belt to maintain the arms’ positioning (Fig. 6.8b). Position the patient’s legs and feet comfortably, and ensure an agreeable room temperature.
Materials
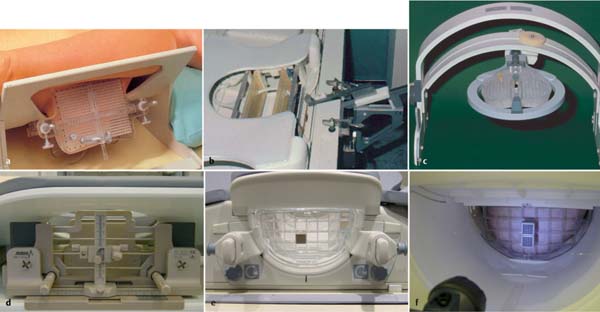
Surface coils and localization equipment. The performance of MRI-guided VAB requires use of an open breast surface coil, which allows access to the breast from the lateral, medial, and/or CC direction. In addition, special needle guide attachments that allow the exact and reliable localization of the puncture coordinates in the x- and y-axes must be used. These are available as biopsy and localization grid systems (some of which can be angled), and continuously variable targeting and puncture systems (post and pillar) (Fig. 6.9). Breast surface coils with ventral breast access are not recommended for performing vacuum-assisted interventions.
Puncture equipment. At present, there are three functional systems available for performing MRI-guided VABs: Fig. 6.10 depicts their clinical applications.
Procedure
Once the indication for MRI-guided percutaneous biopsy has been ascertained in a diagnostic MR mammography examination, the intervention steps are performed as shown in Fig. 6.11.
Fig. 6.8 Positioning and comfort during MR mammography examination.
a Head support with integrated mirror optic.
b Patient positioned with arms in relaxed position, hanging in belt loop.
Fig. 6.9a–f Dedicated MRI-compatible puncture equipment.
a Open breast surface coil with Plexiglas attachment containing multiple puncture channels (Phillips Healthcare, Amsterdam, the Netherlands). Patient lies in oblique position.
b Dedicated unilateral open intervention coil with puncture equipment mounted on edge of examination table (Siemens, Berlin/Munich, Germany).
c Commercial shoulder flex coil with integrated, gadolinium solution-filled puncture components mounted on a C-arm (self-construction; Göttingen University, Germany).
d–f Open diagnostic breast surface coil (GE Healthcare, Chalfont St., Giles, UK): Lateral puncture attachment with post and pillar targeting equipment (Noras MRI Products, Hochberg, Germany) (d). Lateral biopsy and localization grid (Noras MRI Products) (e). Medial biopsy and localization grid (Noras MRI Products) (f).
Fig. 6.10a–f Various needle systems for MRI-guided VAB.
a ATEC biopsy system with appropriate coaxial introduction needle (Suros Surgical Systems, Inc., Indianapolis, IN).
b Retrieval of tissue samples from specimen retrieval chamber.
c Mammotome biopsy system with appropriate coaxial introduction needle (Ethicon Endo-Surgery, Cincinnati, OH).
d Consecutive retrieval of tissue samples from specimen collection window.
e VACORA biopsy system with appropriate coaxial introduction needle (Bard Biopsy Systems, Tempe, AZ).
f Specimen retrieval from biopsy notch.
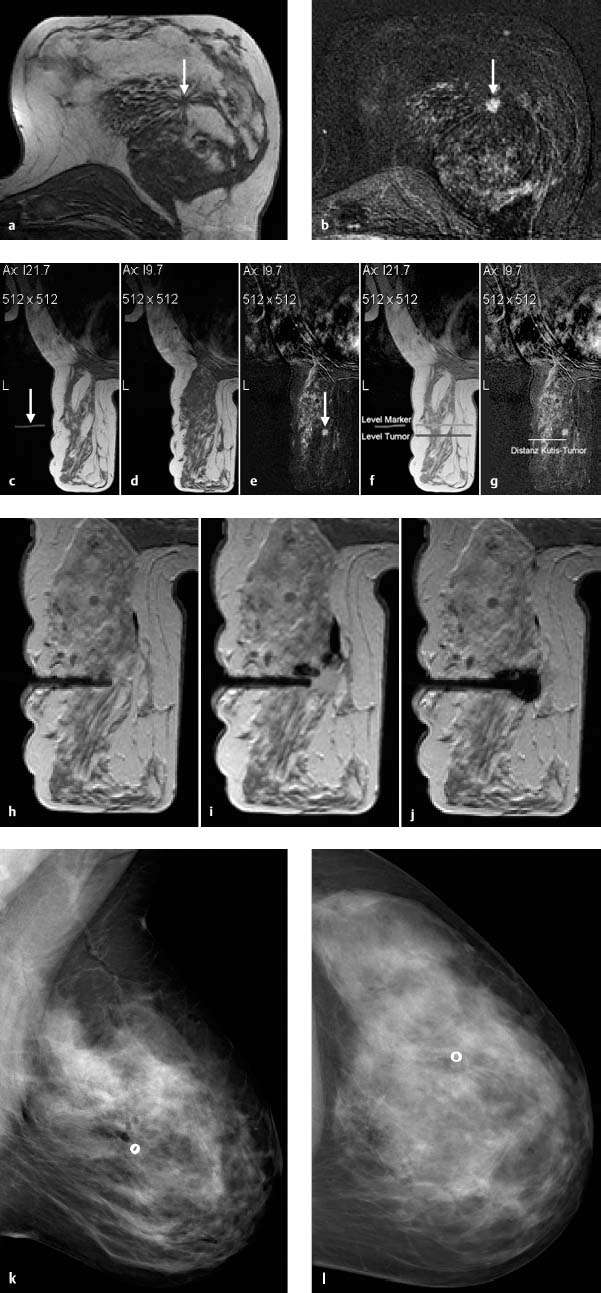
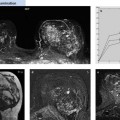
Fig. 6.11a–l Procedure steps performed during MRI-guided VAB. Diagnostic MR mammography shows suspicious lesion in the left breast. Precontrast image (a). Subtraction image (b) (arrows). After positioning the patient for the performance of MRI-guided VAB, an external marker (arrow) with a high signal intensity in the T1-weighted measurements (e. g., containing oil or gadolinium) is placed on the puncture device at the estimated lesion position to serve as the zero-point for calculations (c).
Contrast-enhanced MR mammography is performed prior to VAB. The T1-weighted precontrast image shows the external marker in slice image “Ax I 21.7” (arrow) (c). The target lesion (arrow) is identified in the subtraction image of slice “Ax I 9.7” (e). The corresponding T1-weighted precontrast image (d) is used for comparison with check images during biopsy. The puncture coordinates (x- and y-axes) must now be calculated taking the arbitrary zero-point into consideration, and the depth (z-axis) measured in the appropriate slice. In the case presented here, the puncture site lies 12 mm cranially (y-axis) of the zero-point: the external marker is in slice image “Ax I 21.7,” the target lesion is in slice “Ax I 9.7” (c, e), the puncture position is thus moved this difference of 12 mm in the cranial direction. In the x-axis, the puncture position must be moved ventrally the distance between the level of the marker and the level of the target lesion (10 mm) (f). The depth of the lesion is marked on the monitor from the skin to the lesion and calculated by the computer (g). It is prudent to perform a check MR mammography (T1-weighted series) before puncture and after moving the external marker to the calculated x- and y-coordinates. After disinfection of the skin and application of local anesthesia at the calculated puncture site, the coaxial introduction needle is inserted to the calculated depth (if necessary, perform nick incision). The position of the coaxial introduction needle is checked by performing an MRI T1-weighted series after replacing the puncture stylet with a MRI-compatible mandrin (h). If necessary, correct position and perform an additional position-check.
Once the correct position has been achieved, the biopsy needle is inserted into the coaxial introduction cannula and the specimens are harvested using a vacuum-assisted technique. After the harvesting of specimens is completed, the biopsy needle is removed and another MRI check is performed with the MRI-compatible mandrin in place (i). The additional administration of contrast material at this point is optional. The acquired images should be evaluated to ascertain whether the target lesion has been completely or incompletely removed, or possibly missed. If necessary, further tissue samples should be harvested. It is optional, but sometimes prudent, to place a marker coil/clip in the biopsy cavity through the coaxial introduction cannula (j).
Finally, the coaxial introduction needle can be removed, and the biopsy site compressed, cooled, and bandaged. A postbiopsy mammogram in craniocaudal and mediolateral projection should be performed for documentation (k, l).
Checklist: MRI-guided Vacuum-assisted Biopsy
1. Establish indication.
2. Confirm that there is no correlating finding on mammography and/or (second-look) US.
3. Obtain informed consent (in advance).
4. Position patient comfortably in an open breast surface coil with a biopsy option.
5. Choose the appropriate puncture equipment (grid, post and pillar).
6. Position external marker at estimated target position (arbitrary zero-point).
7. Perform dynamic MR mammography examination with image subtraction.
8. Identify target.
9. Calculate appropriate x-, y-, and z-coordinates.
10. Move external marker to appropriate puncture site and perform MRI check.
11. Disinfect breast skin and administer local anesthesia; if necessary, perform a nick incision.
12. Introduce coaxial introduction needle.
13. Perform MRI check of needle position in relation to target lesion and correct position, if necessary.
14. Demonstrate biopsy sounds.
15. Obtain tissue samples: ≥ 12 tissue samples with 11-gauge needle, or volume equivalent.
16. Perform MRI check of biopsy cavity position in relation to target lesion (possibly with additional contrast administration).
17. If necessary, harvest additional tissue samples.
18. Consider placing marker coil/clip.
19. Remove coaxial introduction needle.
20. Release breast from puncture device while applying compression to puncture site. Move patient to reclining chair, apply compression, and cool puncture site.
21. Perform final mammograms in two orthogonal planes.
Postbiopsy MRI check with contrast administration. It is important to perform an MRI check of the biopsy cavity during the course of or after concluding a diagnostic intervention. This can be performed with or without the additional administration of contrast material, depending on the target lesion. An MRI check without contrast administration is usually sufficient to confirm the biopsy cavity location in relation to nonmass lesions. In the case of a mass-like enhancement, a contrast-enhanced MRI check to document the partial (Fig. 6.12) or complete (Fig. 6.13) removal of the target lesion is recommended. Knowledge of the exact location of residual tumor areas will also facilitate the directed harvesting of further tissue samples when required. When performing a contrast-enhanced MRI check, keep in mind that there is a potential for bleeding into the biopsy cavity, and this should not be confused with residual tumor in the subtraction image (Fig. 6.14).
Quality Control: MRI-guided Percutaneous Biopsy Guidelines*
Indications: MR mammography findings in the MR mammography BI-RADS categories 4 and 5 (occasional exception: MR mammography BI-RADS 3 findings, e. g., when a patient wishes definitive diagnosis), which have no correlating finding on clinical examination or in other imaging modalities (mammography, US).
Equipment: MRI unit with at least 1.0 T
 open breast surface coil allowing access to the breast
open breast surface coil allowing access to the breast
 MRI-compatible localization equipment
MRI-compatible localization equipment
 MRI-compatible puncture equipment:
MRI-compatible puncture equipment:
– VAB (method of choice)
– large core needle biopsy (CNB) (used only in exceptional cases)
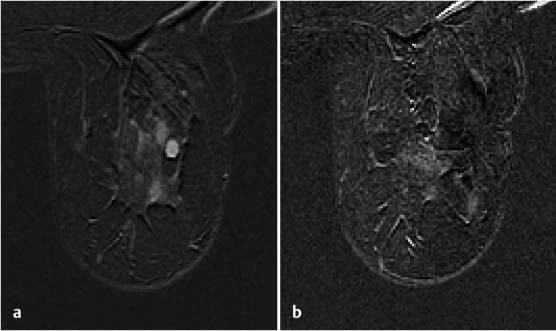
Fig. 6.12a, b Partial removal of enhancing lesion. a Ambiguous oval mass before MRI-guided VAB. b Periinterventional contrast enhanced MR mammography check after harvesting 12 tissue samples. Documentation of a partial removal from the center of the target lesion.
Fig. 6.13a, b Complete removal of enhancing lesion. a Primary finding on MR mammography shows mass lesion in the left breast before MRI-guided VAB. b Contrast-enhanced MR mammography check after harvesting nine tissue samples (8 gauge) shows no more contrast enhancement as confirmation of complete removal.
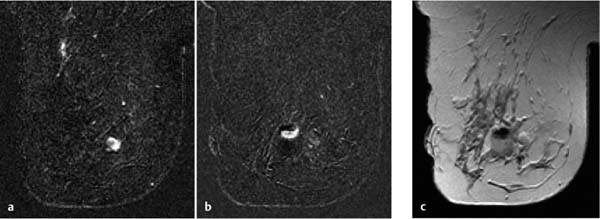
Fig. 6.14a–c False residual tumor. a Hypervascularized mass lesion in diagnostic MR mammography before MRI-guided VAB. b Contrast-enhanced MR mammography check (subtraction image) after harvesting 12 tissue samples shows signal-intense structure that could possibly be falsely interpreted as residual tumor. c In direct comparison with the T1-weighted examination, the signal-intense structure seen in the subtraction image can be localized within the biopsy cavity and represents contrast-containing blood (jet effect).
 If the target lesion is not, or only questionably, reproduced then the intervention should be discontinued and a short-term follow-up examination in 6 months recommended.
If the target lesion is not, or only questionably, reproduced then the intervention should be discontinued and a short-term follow-up examination in 6 months recommended.
 If the target lesion is clearly identified, then the puncture coordinates (x-, y-, and z-axes) are calculated. The calculated puncture site is marked with an external marker (e. g., oil-containing), and a MRI-check is performed without additional contrast administration.
If the target lesion is clearly identified, then the puncture coordinates (x-, y-, and z-axes) are calculated. The calculated puncture site is marked with an external marker (e. g., oil-containing), and a MRI-check is performed without additional contrast administration.
 If the marker position is incorrect, then a new calculation is performed and the marker position corrected. If the marker position is correct, then the skin is disinfected and local anesthesia is administered (if necessary, a skin nick incision). The coaxial introduction needle is positioned (e. g., using the metal puncture stylet) to the appropriate depth (z-axis). The correct position is dependent upon the VAB system being used.
If the marker position is incorrect, then a new calculation is performed and the marker position corrected. If the marker position is correct, then the skin is disinfected and local anesthesia is administered (if necessary, a skin nick incision). The coaxial introduction needle is positioned (e. g., using the metal puncture stylet) to the appropriate depth (z-axis). The correct position is dependent upon the VAB system being used.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree