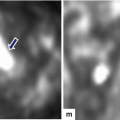
Fig. 7.1
Mucinous cystic neoplasm of the pancreas gross appearance. Photograph (a) shows a distal pancreatectomy and splenectomy specimen with a round encapsulated mass in the tail of the pancreas. Photograph (b) shows a well-defined, round, encapsulated mass with a smooth serosal surface
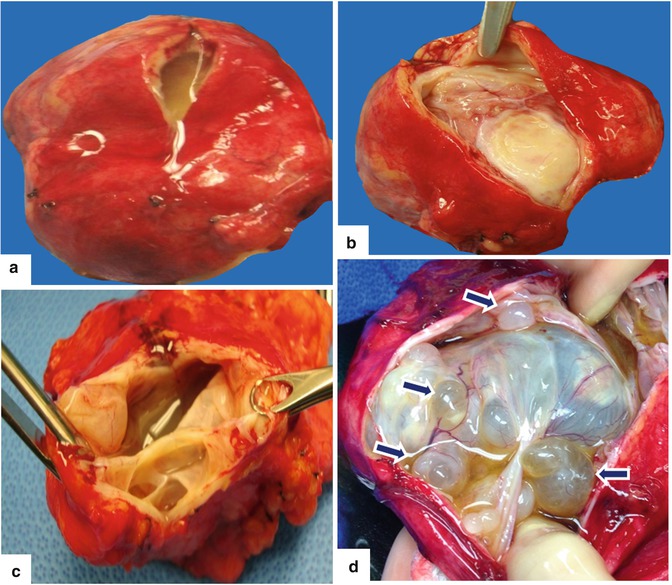
Fig. 7.2
Mucinous cystic neoplasm of the pancreas internal appearance. Photographs (a–c) of bivalved gross specimens demonstrate the variated appearance of cystic neoplasm. (a) Cystic mass with a smooth inner surface and clear mucinous fluid; (b) cystic mass with a thin, smooth, and corrugated inner lining; and (c) trabeculated cystic mass with a thin, smooth capsule and inner lining forming multiple internal septae and (d) multicystic mass with mucinous fluid
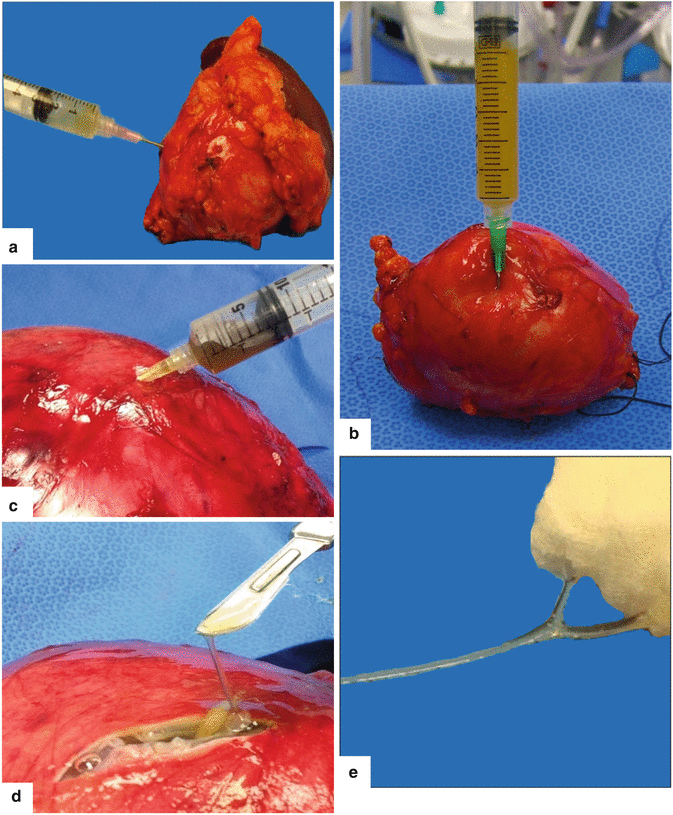
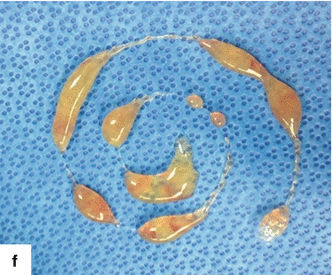
Fig. 7.3
Fluid characteristics on mucinous cystic neoplasm of the pancreas. Photographs of aspirated fluid from three different mucinous cystic neoplasms illustrate the different appearance of the fluid: (a) clear yellow, thin fluid, (b) opaque yellow, and (c) light brown and viscous (d–f). Note the sticky mucoid consistency of the fluid (d–f)
Unilocular or multilocular (20 %) cystic mass
Mean size of 7–8 cm (ranges from 0.5 to 35 cm)
Round smooth contour formed by a thin or thick fibrous capsule
May contain internal septations
Cystic cavity can be filled with thin clear yellow, thin opaque yellow, or light brown mucinous fluid
Necrotic tissue or hemorrhagic material may be present
Calcifications are uncommon (20 %)
Usually in the periphery
Eggshell-like calcifications or punctate calcifications
Occasionally may be present in the internal septa
Signs that suggest malignant transformation
Thick wall with peripheral calcifications
Papillary proliferations
Vascular involvement
7.3.2 Microscopic Appearance
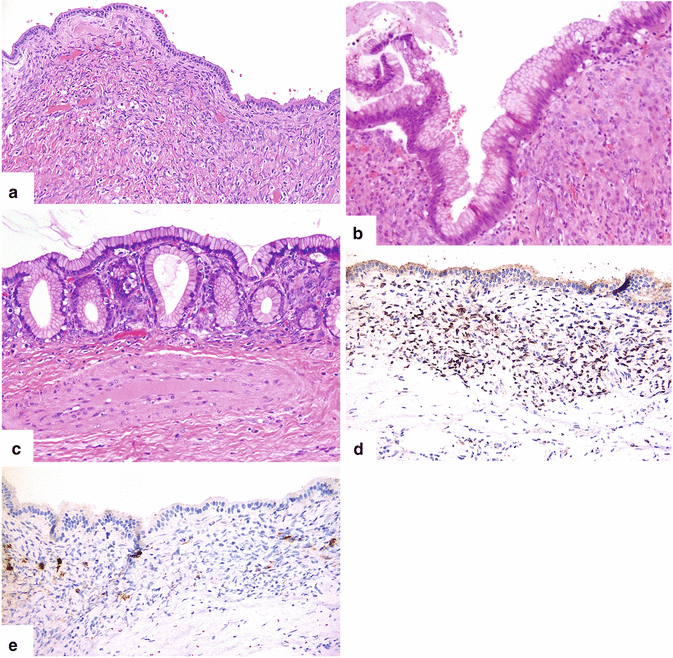
Fig. 7.4
Mucinous cystic neoplasm of the pancreas. Light microscopy demonstrates that the cysts are lined by simple columnar (a) (H&E, 10×), pseudo-stratified columnar (b) (H&E, 20×), and columnar epithelium forming glands (c) (H&E, 20×). The abundant clear cytoplasm and small basally located nuclei indicate a bland cytologic morphology. Note the increased cellularity of the underlying stroma, which is composed of spindle cells that resemble ovarian stroma. These stromal cells are diffusely positive for estrogen receptor (d) and focally positive for inhibin (e) by immunohistochemistry (immunoperoxidase, 20×)
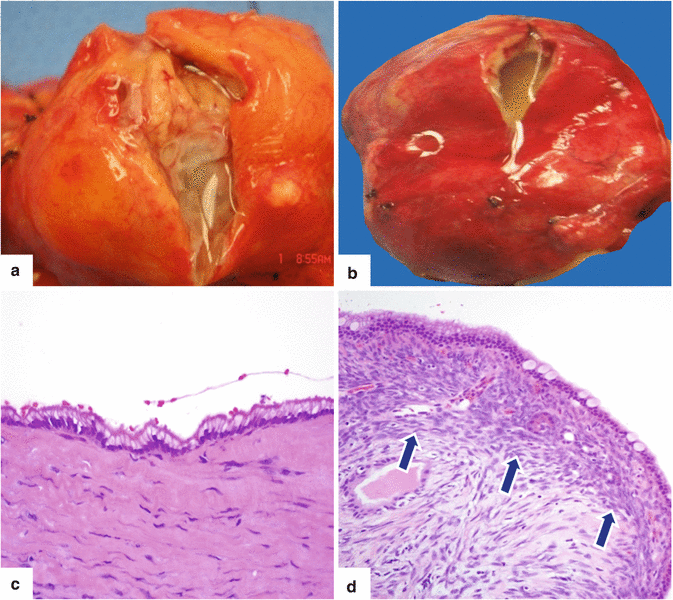
Fig. 7.5
Distinction between mucinous cystic neoplasm (MCN) and intraductal papillary mucinous neoplasm (IPMN). Photographs of two gross specimens, (a) IPMN and (b) MCN, show two grossly similar cystic masses with clear mucinous fluid. Microscopically, the cyst lining of the IPMN (c) (H&E, 20×) is composed of a single-layer epithelium of columnar cells. Dense fibroconnective tissue composed of collagen and fibroblasts makes up the underlying stroma. The epithelium of the MCN (d) is also composed of single layer of columnar cells with few scattered goblet cells adjacent to the characteristic ovarian-type stroma (arrows)
Epithelium
Composed of tall columnar mucin-producing epithelium.
Papillae and complex architectural pattern may be seen.
Epithelial lining is positive for cytokeratin and less frequently positive for CEA, DUPAN-2 MUC-5, and CA19-9.
Histologically, can be subclassified:
Low-grade dysplasia: columnar epithelium with basally located nuclei and apical mucin
Intermediate-grade dysplasia: pseudostratified columnar epithelium with variable mucin content and nuclear atypia
High-grade dysplasia/carcinoma in situ: disorganized, crowded epithelium with atypia, and lack of nuclear polarization and mucin depletion
Malignancy is diagnosed based on cytomorphology, invasion, and metastasis
Subepithelial stroma
Ovarian-type
Spindle cell stroma may contain single epithelioid cells resembling luteinized ovarian hilar cells (positive for inhibin, estrogen (ER), and progesterone (PR) receptors).
The immunophenotype of ovarian-type stroma is similar to normal ovarian stroma with positivity for alpha-inhibin, PR, and ER.
Sarcomatous
Rare
Osteoclast-like giant cells
Rare; few are described in the literature
Practical Pearl
The presence of ovarian-type stroma is mandatory in diagnosing MCN and distinguishing it from an IPMN.
7.4 Clinical Presentation
The majority of MCNs are slow growing and asymptomatic.
Patients may complain of:
Epigastric heaviness and fullness
Abdominal mass
Nausea
Vomiting
Back pain
7.5 Laboratory Evaluation
Typically nonspecific
Serum CA 19–9 and CEA normal
Mucinous neoplasm cyst fluid
Elevated carcinoembryonic antigen (CEA) is the most accurate marker.
CEA concentration has no direct correlation with malignancy.
7.6 Imaging
Preferred imaging modalities
Ultrasound
Contrast-enhanced computed tomography (CECT)
Magnetic resonance (MR)
7.6.1 Ultrasound (Transabdominal, Endoscopic, Intraoperative) (Figs. 7.6–7.11)
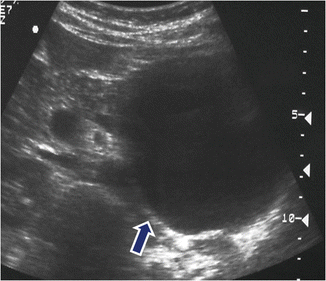
Fig. 7.6
Unilocular mucinous cystic neoplasm on US. A 30-year-old female with left upper quadrant pain. Transverse image shows a round cystic mass with thin walls involving the tail of the pancreas (arrow)
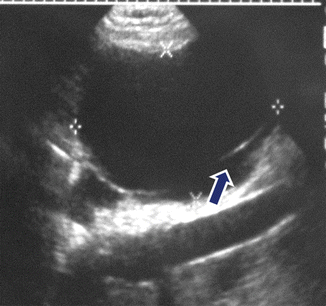
Fig. 7.7
Mucinous cystic neoplasm with thin internal septation on US. A 38-year-old female with abdominal discomfort. Sagittal image demonstrates a large cystic mass with a single internal septation (arrow) involving the body of the pancreas
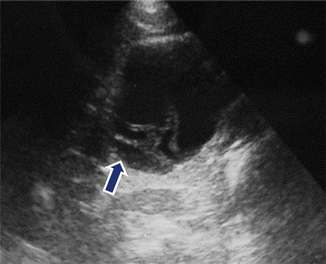
Fig. 7.8
Mucinous cystic neoplasm with thick internal septations on US. Incidental finding on a 45-year-old female patient who had an abdominal ultrasound performed to evaluate for gallstones. Sagittal image reveals a cystic mass with thick internal septations (arrow)
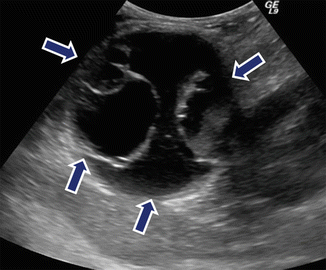
Fig. 7.9
Multilocular mucinous cystic neoplasm of the pancreas on US. A 31-year-old female with abdominal distention. Sagittal image shows a round cystic mass with multiple internal locules of different sizes (arrows) involving the tail of the pancreas
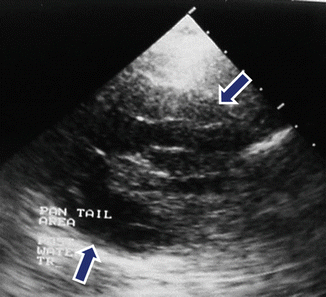
Fig. 7.10
Complex mucinous cystic neoplasm of the pancreas on US. A 43-year-old female with epigastric discomfort. Transverse image demonstrates a complex mass with solid and cystic components (arrows) involving the body of the pancreas
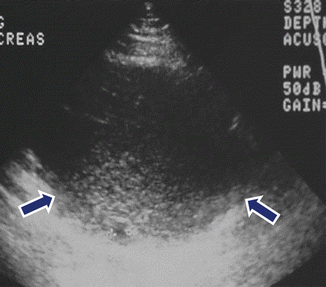
Fig. 7.11
Mucinous cystic neoplasm with internal debris on US. A 51-year-old female with epigastric pain. Transverse image shows a cystic mass with internal low-level echoes (arrows) involving the body and tail of the pancreas
Findings
Ovoid or round unilocular cystic mass in the body or tail of the pancreas
May contain single or multiple internal, thin or thick septations
Cystic mass with internal low-level echoes
Cystic mass with internal papillary projections
Multilocular cystic mass
May contain peripheral calcifications
Color Doppler: avascular cystic mass
7.6.2 Computed Tomography (CT) (Figs. 7.12–7.27)
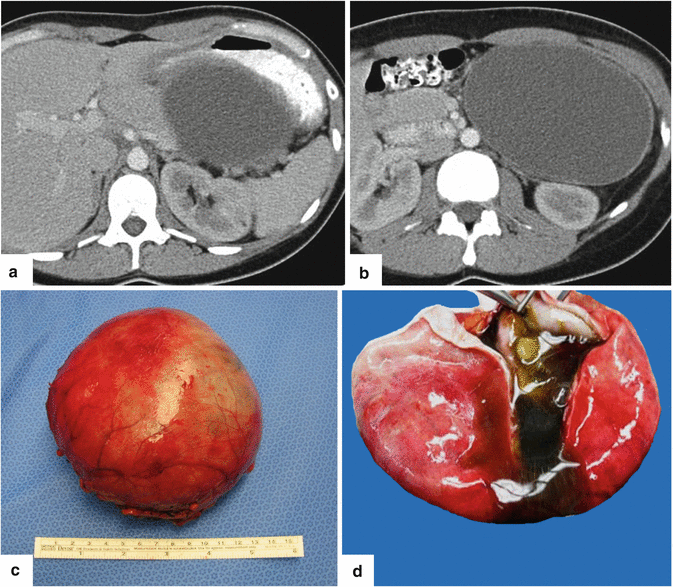
Fig. 7.12
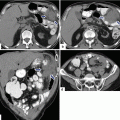
Unilocular mucinous cystic neoplasm on CT. A 33-year-old female who presented with a palpable abdominal mass. CECT axial images (a, b) demonstrate a large cystic mass involving the body and tail of the pancreas. Photograph of the gross specimen (c) demonstrates an ovoid mass with a smooth surface. Photograph of the open specimen (d) demonstrates a cystic mass with smooth lining and containing greenish mucoid fluid
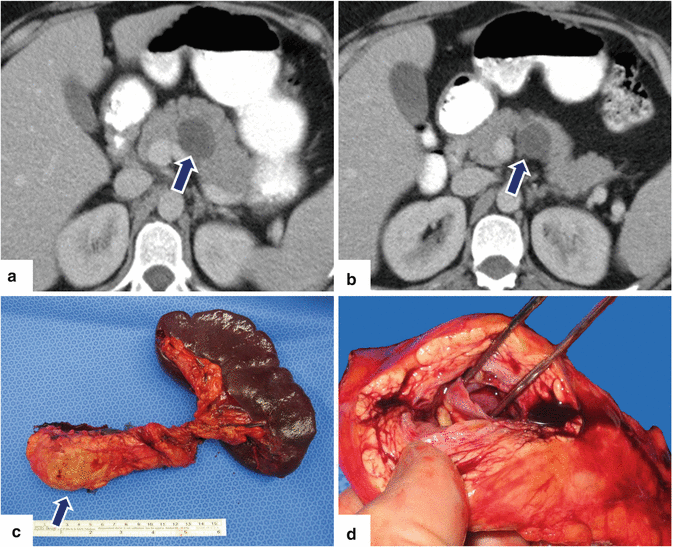
Fig. 7.13
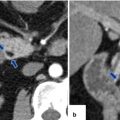
Unilocular mucinous cystic neoplasm of the pancreas on CT. A 30-year-old female with history of uterine fibroids and ovarian cysts was found to have an enlarging cystic lesion in the body of the pancreas with elevated serum CA19-9 and atypical cells with mucin material on endoscopic ultrasound guided fine-needle aspiration. Findings are compatible with a mucinous cystic neoplasm. CECT axial images (a, b) show a small, well-circumscribed cystic mass in the body of the pancreas. Patient underwent a distal pancreatectomy and splenectomy. Photograph of the surgical specimen (c) shows a pancreatic mass with a smooth surface (arrow). Photograph (d) of the pancreatic mass shows a cystic mass with smooth inner lining
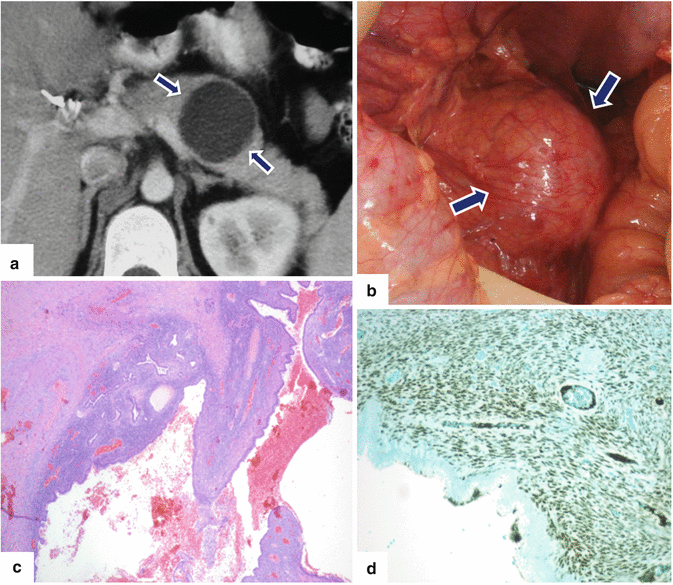
Fig. 7.14
Mucinous cystic neoplasm of the pancreas on CT. A 33-year-old female with a palpable epigastric mass. CECT axial image (a) shows a round cystic mass in the body of the pancreas (arrows). Intraoperative photograph (b) shows a cystic mass with smooth surface (arrows). Histological section (c) (H&E, 4×) illustrates a cystic cavity with hemorrhage lined by columnar epithelium with underlying ovarian-type stroma, which shows estrogen immunoreactivity (d) (immunoperoxidase, 10×)
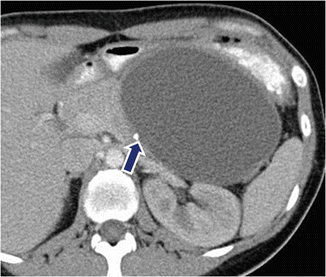
Fig. 7.15
Mucinous cystic neoplasm of the pancreas with peripheral calcification on CT. A 41-year-old female with epigastric pain and early satiety. CECT axial image demonstrates an ovoid cystic mass involving the body and tail of the pancreas with a peripheral punctate calcification (arrow)
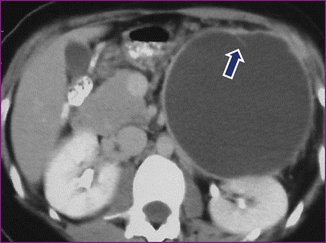
Fig. 7.16
Mucinous cystic neoplasm with septation on CT. A 36-year-old female with an incidental finding on CT. CECT shows a cystic mass with a thin wall and a single internal septation (arrow) involving the tail of the pancreas
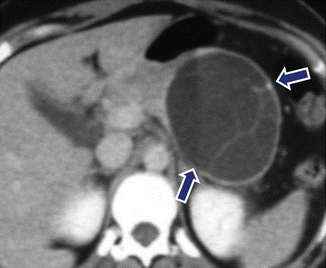
Fig. 7.17
Mucinous cystic neoplasm with internal septations on CT. A 26-year-old female with epigastric fullness. CECT axial image reveals a cystic mass with internal thin septations (arrows) involving the body of the pancreas
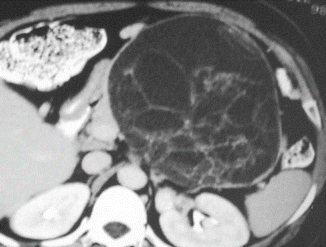
Figs. 7.18
Mucinous cystic neoplasm with internal septations on CT. A 38-year-old female with palpable mass in the left upper quadrant. CECT axial image reveals a cystic mass with multiple, internal thin and thick septations
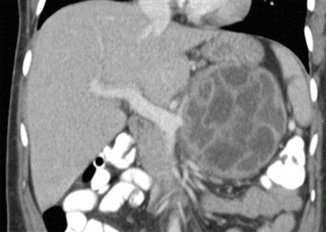
Fig. 7.19
Mucinous cystic neoplasm of the pancreas with thick septations on CT. A 26-year-old female with epigastric fullness. CECT coronal image shows a round mass with multiple internal thick septations involving the body of the pancreas
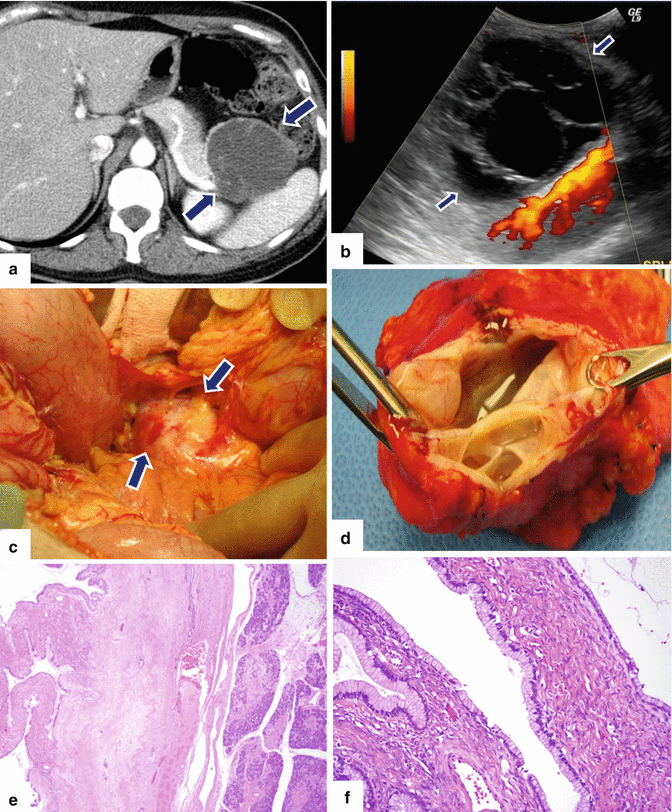
Fig. 7.20




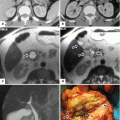
Symptomatic small mucinous cystic neoplasm of the pancreas on CT. A 49-year-old female with LUQ pain radiating to her back for 6 months. No history of pancreatitis, prior trauma, or injury. CECT axial image (a) shows a multiseptated, avascular, cystic mass in the tail of the pancreas (arrows). Intraoperative ultrasound (power Doppler) (b) confirms a multiseptated pancreatic mass (arrows). Intraoperative photograph (c) shows a mass in the tail of the pancreas with smooth surface (arrows). Photograph of the inner surface of the cyst (d) shows multiple septations. Histological section (e) (H&E, 4×) shows the interphase between pancreatic parenchyma and the cystic tumor. At higher magnification (f) (H&E, 20×), the simple columnar mucinous epithelium and ovarian-type stroma are better appreciated
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree