Neuroendocrine tumors are rare solid tumors with an estimated 12,000 people in the United States diagnosed each year. Neuroendocrine tumors can occur in any part of the body. There is a wide spectrum of disease, ranging from slow-growing and indolent tumors found incidentally to highly aggressive malignancies with a poor prognosis. Knowledge of neuroendocrine tumor pathology is essential in the diagnostic workup of these patients. This article focuses on the evaluation, detection, and staging of common neuroendocrine tumors with multiple imaging modalities; the information gained with a multimodality approach is often complementary and leads to image-guided treatment decision making.
Key points
- •
Neuroendocrine tumors are a heterogeneous group of tumors and knowledge of pathology is essential to selecting the appropriate imaging modality.
- •
DOTATATE-PET/computed tomography scans offer substantial improvement for systemic staging of well-differentiated neuroendocrine tumors compared with computed tomography scans and MR imaging and offer potential value for theranostic applications.
- •
Neuroendocrine tumors can occur as part of systemic syndromes, such as multiple endocrine neoplasia, and detection of 1 neoplasm may prompt additional screening for other neuroendocrine neoplasms.
Introduction
Neuroendocrine tumors (NET) are a rare type of solid tumor with an estimated 12,000 people in the United States diagnosed with a NET each year and approximately 170,000 people living with a NET. In the past several decades, the incidence rate of NETs has continued to increase, with a 6.4-fold increase between 1973 and 2012. This increase is thought to be in part owing to an increased awareness of NETs, but largely attributed to improvements medical imaging and endoscopy (including endoscopic ultrasound). There is a wide spectrum of disease in NETs, ranging from slow-growing and indolent tumors that are incidentally found on imaging for unrelated clinical indications to highly aggressive malignancies with a poor prognosis. Despite the increase in the prevalence and detection of NETs, significant improvements in overall survival have been observed since 2000. In 2018, the US Food and Drug Administration approved a targeted systemic therapy to somatostatin receptor (SSTR)-expressing gastroenteropancreatic NETs (GEP-NETs). The focus of this article is on the evaluation, detection, and staging of the most common types of NETs with multiple imaging modalities, because the information gained with a multimodality approach is often complementary and leads to image-guided treatment decision making at a patient-specific level. Additionally, given the spectrum of pathology in NETs, the article briefly addresses key pathologic issues that guide imaging evaluation in patients with NETs.
Imaging protocols
Computed Tomography Scans and MR Imaging
The current American College of Radiology Appropriateness Criteria for neuroendocrine imaging focuses only on pituitary imaging, which are covered in a different chapter of this book. However, no American College of Radiology Appropriateness Criteria exist to describe appropriate imaging of NETs in the chest, abdomen, and pelvis. Multidetector computed tomography (CT) scanning is widely used in the assessment of pancreatic NETs with detection rates of up to 69% to 94%. On dual phase pancreatic CT protocols used to evaluated suspected pancreatic masses, NETs are typically hypervascular and demonstrate avid enhancement in the arterial phase. This factor is especially important in the diagnosis of liver metastases from NET. Some studies have shown that the contrast enhancement pattern of NETs can correlate with the tumor grade. Cappelli and colleagues showed that tumors with venous and delayed phase enhancement are likely to be high grade compared with tumors with arterial enhancement and venous phase washout. Other findings, such as tumor size, ill-defined tumor margins, pancreatic duct dilation, and vascular invasion, have also been found to be significant in predicting grading of tumors. Dual energy CT scans can help to improve the detection of small pancreatic NETs, particularly using low-energy monochromatic and iodine density images. For assessment of small bowel NETs, CT scans and MR enterography protocols are used with unenhanced images, followed by arterial and venous phase acquisitions after distension of the bowel by neutral contrast. Recent studies have shown that CT scans and MR enterography have higher sensitivity (100% and 86%–94%, respectively) and specificity (96.2% and 95%–98%, respectively) for the detection of small bowel neoplasms, including NETs. ,
Owing to its superior soft tissue characterization, MR imaging has an improved detection rate compared with CT, particularly for the characterization of previously indeterminate pancreatic lesions. Additional sequences such as diffusion-weighted imaging and apparent diffusion coefficient mapping can help to localize and potentially grade the tumors. MR cholangiopancreatography is useful to evaluate the status of pancreatic duct and biliary system and should be performed for surgical planning. Other focal pancreatic lesions like hypervascular metastasis, intrapancreatic accessory splenule, or serous cystadenoma can be sometimes be confused with NETs, and MR imaging can aid with problem solving in such cases. ,
[ 68 Ga]DOTATATE PET/Computed Tomography Scans and PET/MR Imaging
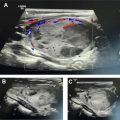
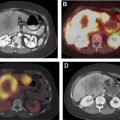
[ 68 Ga]DOTATATE was approved in 2016 for the localization of SSTR-positive NETs in adult and pediatric patients. Once injected, DOTATATE binds to SSTRs on the cell surface, with highest affinity for the SSTR2 receptor. Clinically, [ 68 Ga]DOTATATE-PET/CT is rapidly becoming the standard of care in the evaluation of NET, owing to its superior sensitivity and specificity for detection of metastatic disease when compared with [ 111 In]pentetreotide scintigraphy. Frequently, the findings from a [ 68 Ga]DOTATATE-PET/CT are complementary to CT and MR imaging, which offer higher spatial resolution and aid in surgical planning and decision-making. As peptide receptor radionuclide therapy (PRRT) with [ 177 Lu]DOTATATE becomes increasingly available and offered to patients, [ 68 Ga]DOTATATE-PET/CT is essential to assess patient eligibility before PRRT. Finally, as the emerging hybrid imaging modality PET/MR imaging becomes increasingly available, [ 68 Ga]DOTATATE-PET/MR imaging allows for synchronous acquisition of both PET and MR imaging data with excellent anatomic co-registration and may allow for simultaneous assessment of metastatic disease using both [ 68 Ga]DOTATATE-PET and diagnostic contrast-enhanced MR imaging of the abdomen ( Fig. 1 ).
[ 68 Ga]DOTATATE-PET scans require preparation of the radiopharmaceutical before injection into the patient. The radionuclide [ 68 Ga] is eluted from a [ 68 Ge] generator and has a half-life of 68 minutes, which somewhat limits its ability to be commercially distributed compared with [ 18 F], which has a half-life of 110 minutes. The recommended dose of [ 68 Ga]DOTATATE is 2 MBq/kg (0.054 mCi/kg) up to 200 MBq (5.4 mCi). Patients are instructed to be well-hydrated before performance of the PET scan and are advised to void frequently after injection of radiotracer to decrease radiation exposure. Approximately 40 to 90 minutes following injection of [ 68 Ga]DOTATATE, patients can be scanned with a typical field-of-view from skull base to the mid thigh.
[ 111 In]Pentetreotide Scintigraphy
Before the approval of [ 68 Ga]DOTATATE for imaging of NETs, [ 111 In]pentetreotide (Octreoscan; Covidien Inc., Dublin, Ireland) was the standard of care for molecular imaging of NETs. The mechanism of action of pentetreotide is the binding of SSTRs at the cell surface, albeit with a lower affinity than [ 68 Ga]DOTATATE. Additionally, owing to the gamma emission of 171 keV and 245 keV photons from [ 111 In] during its decay, imaging is limited to planar and single photon emission CT (SPECT) or SPECT/CT images on a gamma camera. The half-life of [ 111 In] is 2.8 days, and images obtained during [ 111 In]pentetreotide scintigraphy are routinely acquired at 24 hours after injection, with optional imaging at 4 and 48 hours after injection. This nature leads to patients having to return to the nuclear medicine department multiple times, which is a significant drawback when compared with [ 68 Ga]DOTATATE-PET/CT. Although an important historical radiotracer that provided key diagnostic information about NETs, current use of [ 111 In]pentetreotide is limited to practice settings with limited or no access to [ 68 Ga]DOTATATE and has been shown to be an inferior molecular imaging agent.
Pathology and imaging considerations
Variable Somatostatin Receptor Expression Among Neuroendocrine Tumors
It is well-known that NETs are a heterogeneous group of tumors and that certain NETs overexpress different SSTR subtypes at the cell surface. Historically, [ 111 In]pentetreotide scintigraphy preferentially localized to the SSTR2 and SSTR5 receptors, which are upregulated in pheochromocytomas, gastrinomas, glucagonomas, and nonfunctional GEP-NETs. However, both insulinomas and medullary thyroid cancer demonstrate variable levels of SSTR expression, particularly the SSTR2 receptor. , As a result, studies of [ 111 In]pentetreotide scintigraphy demonstrated low sensitivity for the detection of medullary thyroid cancer and insulinomas. [ 68 Ga]DOTATATE-PET/CT scanning has demonstrated significant improvement in detection of insulinomas owing to the higher cell receptor binding and spatial resolution of the PET radiotracer. Thus, when performing a molecular imaging study to evaluate for either primary or metastatic NET, it is essential that the interpreting physician know the suspected pathology of the tumor and the potential limitations of the examination, particularly if performing [ 111 In]pentetreotide scintigraphy. Additionally, certain NETs (such as gastrinoma) occur in characteristic anatomic locations (eg, the gastrinoma triangle), and providing this information to the interpreting physician can allow for greater scrutiny of the scan in the areas of concern.
Neuroendocrine Tumor Differentiation and Choice of Molecular Imaging Agent
Another key pathologic consideration in molecular imaging of NETs is the degree of pathologic differentiation, because it may influence the choice of molecular imaging agent. NETs are classified by the 2017 World Health Organization into 3 pathologic grades (G1–G3) on the basis of the number of mitoses seen on high-powered microscopy, the Ki-67 index, and the presence or absence of tumor necrosis and apoptosis. As an example, G1 NETs are well-differentiated with extremely low Ki-67 (<3%), low number of mitoses, and rare tumor necrosis. The 2017 World Health Organization criteria also introduced a separate subtype of poorly differentiated neuroendocrine carcinoma, which also has large cell and small cell variants, and renamed mixed neuroendocrine or non-neuroendocrine neoplasms. In regard to the selection of PET radiotracers, knowledge of the pathology is paramount, because more aggressive tumors may be better imaged with PET scanning with [ 18 F]fluorodeoxyglucose (FDG-PET)/CT scanning rather than [ 68 Ga]DOTATATE-PET/CT, owing to their more aggressive cellular behavior and poor differentiation, which together result in decreased expression of SSTRs at the cell surface. In cases of G2 NETs, both [ 18 F]FDG-PET/CT scans and [ 68 Ga]DOTATATE-PET/CT scans may be performed before therapeutic intervention, as differential uptake on these 2 examinations may influence therapeutic management between PRRT, systemic chemotherapy, and/or surgery. Thus, interpreting physicians should take caution when interpreting [ 68 Ga]DOTATATE-PET/CT without pathologic data, because low expression in a known NET or suspected metastasis could represent a poorly differentiated NET and may require a [ 18 F]FDG-PET/CT for adequate staging ( Fig. 2 ).
Gastroenteropancreatic neuroendocrine tumors
The gastrointestinal tract and pancreas are the most common locations (about 70%) for NETs. GEP-NETs account for about 1.5% to 2.0% of all primary gastrointestinal and pancreatic neoplasms, and their incidence in the United States is estimated at 3.56 per 100,000 population. The majority of these cases are sporadic, and a small percentage occur in patients with genetic syndromes such as multiple endocrine neoplasia type 1 (MEN-1), neurofibromatosis type 1, Von Hippel-Lindau disease, and tuberous sclerosis complex.
Pancreatic NETs account for about 1% to 2% of all pancreatic neoplasms with an incidence of less than 1%, al though increasing over the last 20 to 30 years. Functioning pancreatic NETs are diagnosed earlier because of the symptoms, but are less common (10%–30%) than nonfunctioning tumors. Among functional NETs, insulinomas are the most common tumors, followed by gastrinomas. The nonfunctioning tumors produce nonspecific symptoms. Because of slow growth and late detection, these tumors can have an advanced stage when diagnosed. , In patients with functioning NETs, laboratory analyses of the specific hormone levels are often diagnostic and can be tested in urine or serum. Chromogranin A is the most commonly used serum marker for diagnosis and is found to be elevated in 60% to 80% patients. ,
The gastrointestinal tract is the most common site of NETs (67%); the distal part of the ileum being the most common location. The majority of ileal NETs are hormonally inactive and can present as vague abdominal pain, gastrointestinal bleeding, or bowel obstruction. Classic carcinoid syndrome (diarrhea, tachycardia, hot flushes, and skin reddening) is seen in 6% to 30% of patients and more commonly occurs (about 95% of cases) with hepatic metastasis. Gastric NETs are rare and account for approximately 0.3% of gastric tumors. Three distinct types of gastric NETs have been described, of which type I and II are asymptomatic and generally manifest as multiple small polyps in gastric fundus and body, incidentally diagnosed on endoscopy ( Fig. 3 ). Type III gastric NETs demonstrate marked enhancement and can have an infiltrative appearance on imaging. Duodenal NETs are most commonly gastrinomas, and one-third of these tumors develop Zollinger-Ellison syndrome owing to excess gastrin secretion. Multiple gastrinomas are more commonly seen in patients with MEN-1 syndrome.
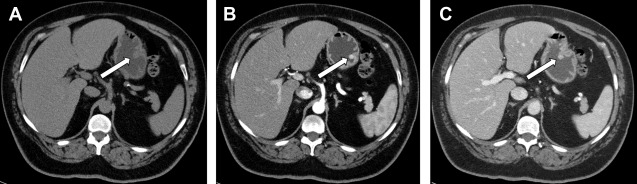
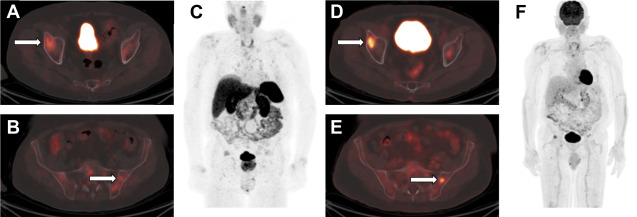
Functioning NETs are usually smaller in size (1–2 cm), well-defined, and hypervascular ( Fig. 4 ). Insulinomas are typically solitary and smaller compared with other functioning NETs. Gastrinomas are located in the gastrinoma triangle, which is bounded by the cystic duct junction with the common bile duct, the pancreatic neck, and the junction of the second and third portions of the duodenum; 60% tumors found in pancreas, and remainder are seen in duodenum or peripancreatic lymph nodes. Those within the duodenum are usually multiple, subcentimeter in size, and better detected by endoscopic ultrasound imaging rather than CT scans or MR imaging.
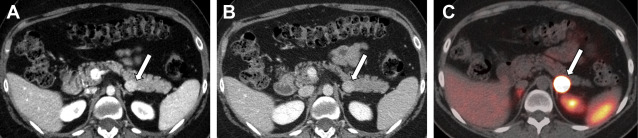
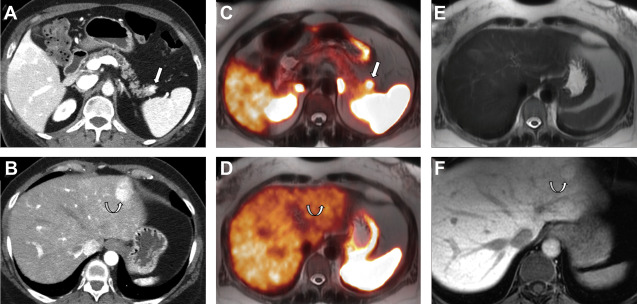
Nonfunctioning NETs are usually larger in size, may be symptomatic owing to the mass effect, have encapsulated margins, and show heterogeneous enhancement, often characterized by areas of cystic degeneration, necrosis, or sometimes fibrosis ( Fig. 5 ). Cystic degenerated NETs are seen in up to 17% of cases, and more commonly in patients with MEN-1 syndrome. The presence of a hypervascular rim on CT scanning or MR imaging can help to suggest the diagnosis compared with other cystic pancreatic masses. Both functioning and nonfunctioning NETs very rarely involve the main pancreatic duct to cause duct dilation, a finding commonly seen in patients with pancreatic adenocarcinoma. Pancreatic NETs usually metastasize to liver and regional lymph nodes, whereas locally invasive tumors can extend into the retroperitoneum.
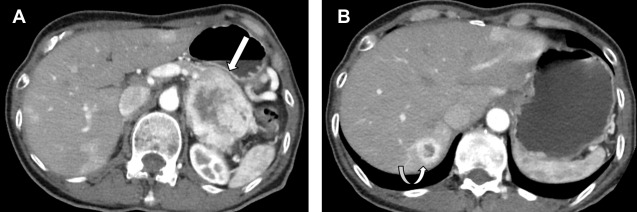
On CT scans and MR imaging, small bowel NETs may present as polypoid hypervascular masses or focal concentric bowel wall thickening. More often, NETs are diagnosed by detection of a mesenteric mass with a surrounding desmoplastic reaction owing to local effects of serotonin. Smaller NETs of the small bowel are difficult to diagnose and may present with only metastatic disease. Appendiceal NETs are incidentally discovered during appendicectomy in 70% cases, have a favorable prognosis compared with other NETs, and metastasize less frequently. As with other GEP-NETs, appendiceal NETs are seen as small arterial hyperenhancing lesions on CT scans and MR imaging and are typically located in the appendiceal tip. Uncommonly, NETs may arise in the colon and rectum, accounting for about 11% of all GEP-NETs. The majority of these tumors are incidentally detected on colonoscopy, but occasionally patients may present with gastrointestinal bleeding and pain.
Nodal staging of NETs is usually performed by CT scan, MR imaging, or PET imaging. Like the primary lesions, nodal metastases are also typically hypervascular and more conspicuous on arterial phase. Liver metastases are also typically hypervascular in arterial phase; lesions with hypoenhancement in all phases suggest a poor prognosis. MR imaging is a more sensitive modality compared with CT scans for the detection of NET liver metastases. Dynamic contrast-enhanced MR imaging, in particular using gadoxetic acid and diffusion-weighted imaging, is helpful in increasing diagnostic confidence for liver metastasis detection.
Somatostatin receptor imaging has long been a part of the diagnosis and staging for GEP-NETs. The initial experience with [ 111 In]pentetreotide demonstrated a similar sensitivity, specificity, and accuracy between helical CT scans and somatostatin receptor scintigraphy. The performance of [ 111 In]pentetreotide somatostatin receptor scintigraphy is increased when performed as an SPECT/CT scans compared with SPECT scans or planar scintigraphy alone. However, given advances in CT scans and MR imaging technology and acquisition techniques, CT scanning and MR imaging now perform better than [ 111 In]pentetreotide, and the use of somatostatin receptor scintigraphy should be limited to cases in which disease is occult on CT or MR imaging. More recently, US Food and Drug Administration approval of [ 68 Ga]DOTATATE and [ 177 Lu]DOTATATE has substantially altered the imaging diagnosis, management, and treatment of GEP-NETs. Multiple studies have shown superior performance of [ 68 Ga]DOTATATE-PET/CT scans compared with [ 111 In]pentetreotide somatostatin receptor scintigraphy, resulting in changes in treatment plans in up to 36% of patients who underwent both scans. , , Given the superior performance of [ 68 Ga]DOTATATE-PET/CT scans among molecular imaging agents and the potential for targeted molecular therapy with [ 177 Lu]DOTATATE PRRT, this approach is the current favored method of systemic staging for patients with NETs before treatment.
Lung and thymic carcinoid tumors
Lung carcinoid tumors account for approximately 1% to 2% of all lung cancers, with approximately 2000 to 4500 newly diagnosed cases in the United States each year. Lung carcinoid tumors are classified as typical or atypical, with typical lung carcinoid tumors arising in slightly younger patients (the average age at diagnosis is 45 years). Lung carcinoid tumors are generally classified as well-differentiated NETs and are often slow growing with a benign clinical courses. Frequently, lung carcinoid tumors are endobronchial lesions that present with symptoms of cough, hemoptysis, or pneumonia secondary to bronchial obstruction from the lesion. Thymic carcinoid tumors (also referred to as NETs of the thymus) are rare tumors, accounting for only 2% to 5% of thymic tumors and 0.4% of all carcinoid tumors, with an estimated incidence of 0.2 per million in the United States. , Thymic NETs are associated with the genetic syndrome MEN-1. Thymic carcinoid tumors have heterogeneous clinical behaviors and pathologic appearances, ranging from asymptomatic and nonaggressive to symptomatic and highly aggressive. If symptomatic, patients often present secondary to symptoms of mass effect or invasion of the mediastinal structures; carcinoid syndrome is an uncommon clinical presentation. , Interestingly, up to 50% of patients with thymic carcinoid tumors have hormonal abnormalities, the most frequent being Cushing syndrome secondary to primary tumoral secretion of adenocorticotropic hormone. Up to 30% of patients with thymic carcinoid present with advanced-stage disease, much higher than lung carcinoid tumors.
Given the general benign course of many lung carcinoid tumors, these lesions are frequently incidentally detected on CT scans of the chest performed for other reasons. Approximately 60% to 70% of lung carcinoids arise in the central airways and involve the main, lobar, or segmental bronchi. These centrally located tumors are more frequently typical carcinoid tumors, but the imaging features of both typical and atypical lung carcinoid tumors overlap and are too similar for confident differentiation on CT scan. Chest radiography often shows a well-defined hilar or perihilar mass with or without the presence of distal airspace disease. When carcinoid tumors arise distal to the segmental bronchi in the lung, these are termed peripheral carcinoids and are more frequently atypical on histology. These carcinoid tumors are typically appear as a well-circumscribed slightly lobulated spherical or ovoid nodule or mass with the long axis parallel to adjacent bronchi or pulmonary artery branches. On CT scans, up to 30% of lung carcinoid tumors demonstrate punctate or diffuse calcifications and may demonstrate avid vascularity and internal enhancement. For endobronchial carcinoid tumors, evaluation with thin section CT chest scanning is useful to establish the relationship between the lesion and the bronchi, and can aid in directing bronchoscopic evaluation and biopsy ( Fig. 6 ). Mediastinal and hilar adenopathy is a common finding on CT scans and may be reactive secondary to recurrent pneumonia versus lymph node metastases, the latter being more common with atypical carcinoids. , Thymic carcinoids arise in the anterior mediastinum and on CT scanning may mimic a thymoma. The appearance of thymic carcinoid is overall nonspecific, but these masses tend to be large, ranging from 6 to 20 cm, and demonstrate heterogeneous enhancement and locally aggressive features. Scattered calcifications may be present within thymic carcinoids. MR imaging is seldom used for the evaluation of pulmonary nodules and masses, but may be used in the evaluation of mediastinal or thymic lesions to assist in the characterization of the internal components. Small case series report that primary thymic NETs are isointense to mildly hyperintense to skeletal muscle on T1-weighted images and heterogeneously T2 hyperintense.