Imaging
EMILY N. VINSON  CHAPTER EDITOR
CHAPTER EDITOR
EMILY N.
VINSON
HISTORY
A 40-year-old woman with diffuse bone pain and a chronic disease.
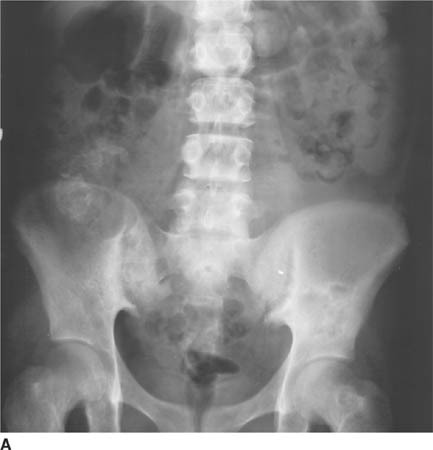
 FIGURE 5-1A Anteroposterior radiograph of lumbar spine and pelvis. There is increased density of the bones. There is a partially calcified renal transplant overlying the right hemipelvis. Small lucent lesions are seen in the bilateral ilia and proximal left femur. There are erosive changes and subchondral sclerosis of the bilateral sacroiliac joints, with ill-defined cortical margins, consistent with subchondral bone resorption.
FIGURE 5-1A Anteroposterior radiograph of lumbar spine and pelvis. There is increased density of the bones. There is a partially calcified renal transplant overlying the right hemipelvis. Small lucent lesions are seen in the bilateral ilia and proximal left femur. There are erosive changes and subchondral sclerosis of the bilateral sacroiliac joints, with ill-defined cortical margins, consistent with subchondral bone resorption.
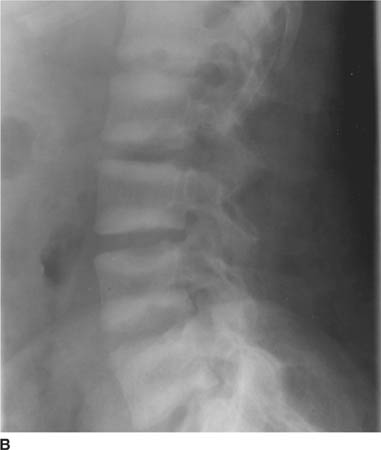
 FIGURE 5-1B Lateral radiograph of lumbar spine. Bandlike areas of sclerosis are noted to involve the vertebral body marrow adjacent to the superior and inferior endplates. Erosive changes are present at the discovertebral junctions of multiple vertebral body endplates, consistent with subchondral bone resorption.
FIGURE 5-1B Lateral radiograph of lumbar spine. Bandlike areas of sclerosis are noted to involve the vertebral body marrow adjacent to the superior and inferior endplates. Erosive changes are present at the discovertebral junctions of multiple vertebral body endplates, consistent with subchondral bone resorption.
DIFFERENTIAL DIAGNOSIS
 Osteopetrosis: Diffuse sclerosis results in a bone-within-bone appearance or a sandwich vertebra appearance. Anterior vascular notches may be seen in the vertebral bodies, but subchondral bone resorption and lytic lesions do not occur, making this an unlikely diagnosis.
Osteopetrosis: Diffuse sclerosis results in a bone-within-bone appearance or a sandwich vertebra appearance. Anterior vascular notches may be seen in the vertebral bodies, but subchondral bone resorption and lytic lesions do not occur, making this an unlikely diagnosis.
 Systemic mastocytosis: Osteosclerosis may be diffuse or patchy and multifocal. Multiple lytic lesions can also occur, which are usually surrounded by a halo of sclerosis. Subchondral bone resorption is not a typical characteristic of this disease.
Systemic mastocytosis: Osteosclerosis may be diffuse or patchy and multifocal. Multiple lytic lesions can also occur, which are usually surrounded by a halo of sclerosis. Subchondral bone resorption is not a typical characteristic of this disease.
 Renal osteodystrophy and secondary hyperparathyroidism: Osteosclerosis manifests as a “rugger-jersey spine” appearance. Subperiosteal, subchondral, and subligamentous bone resorption simulating erosions, and lytic brown tumors are additional features. The presence of a partially calcified renal transplant confirms that this patient has chronic renal disease. This is the most likely diagnosis.
Renal osteodystrophy and secondary hyperparathyroidism: Osteosclerosis manifests as a “rugger-jersey spine” appearance. Subperiosteal, subchondral, and subligamentous bone resorption simulating erosions, and lytic brown tumors are additional features. The presence of a partially calcified renal transplant confirms that this patient has chronic renal disease. This is the most likely diagnosis.
 Myelofibrosis: A diffuse increase in bone density is most commonly seen, but small areas of relative radio-lucency or lytic lesions can also be present. Subchondral bone resorption is not characteristic of this disease process, and this disease does not usually manifest as a “rugger-jersey spine.”
Myelofibrosis: A diffuse increase in bone density is most commonly seen, but small areas of relative radio-lucency or lytic lesions can also be present. Subchondral bone resorption is not characteristic of this disease process, and this disease does not usually manifest as a “rugger-jersey spine.”
DIAGNOSIS
Renal osteodystrophy and secondary hyperparathyroidism
KEY FACTS
Clinical
 Musculoskeletal manifestations of chronic renal insufficiency are increasingly common due to prolonged survival with hemodialysis.
Musculoskeletal manifestations of chronic renal insufficiency are increasingly common due to prolonged survival with hemodialysis.
 Symptomatic bone disease may consist of pain, tenderness, swelling, and deformity.
Symptomatic bone disease may consist of pain, tenderness, swelling, and deformity.
 The two main mechanisms of renal osteodystrophy (also called uremic osteopathy) are secondary hyperparathyroidism and vitamin D deficiency.
The two main mechanisms of renal osteodystrophy (also called uremic osteopathy) are secondary hyperparathyroidism and vitamin D deficiency.
 Secondary hyperparathyroidism in patients with chronic renal disease is caused by a combination of hypocalcemia (due to elevated serum phosphate levels, skeletal resistance to calcium mobilization by parathyroid hormone, and decreased intestinal absorption of calcium caused by decreased vitamin D levels) and decreased renal degradation of parathyroid hormone.
Secondary hyperparathyroidism in patients with chronic renal disease is caused by a combination of hypocalcemia (due to elevated serum phosphate levels, skeletal resistance to calcium mobilization by parathyroid hormone, and decreased intestinal absorption of calcium caused by decreased vitamin D levels) and decreased renal degradation of parathyroid hormone.
 As renal cell mass decreases in moderate and advanced renal disease, so does the body’s ability to synthesize the physiologically active form of vitamin D. In addition, elevated serum phosphate levels inhibit vitamin D production. This leads to skeletal changes of osteomalacia, rickets, or both.
As renal cell mass decreases in moderate and advanced renal disease, so does the body’s ability to synthesize the physiologically active form of vitamin D. In addition, elevated serum phosphate levels inhibit vitamin D production. This leads to skeletal changes of osteomalacia, rickets, or both.
Radiologic
 Radiographic abnormalities in renal osteodystrophy reflect both hyperparathyroidism and vitamin D deficiency, and include bone resorption, brown tumors, bone sclerosis, osteomalacia, osteoporosis, and soft tissue and vascular calcifications.
Radiographic abnormalities in renal osteodystrophy reflect both hyperparathyroidism and vitamin D deficiency, and include bone resorption, brown tumors, bone sclerosis, osteomalacia, osteoporosis, and soft tissue and vascular calcifications.
 Bone resorption is typically subperiosteal, subchondral, and subligamentous in distribution. Characteristic sites of subperiosteal resorption include the radial margins of the middle phalanges of the hands and the medial margins of the proximal tibiae. Characteristic sites of subchondral resorption include the sacroiliac joints, pubic symphysis, and acromioclavicular joints.
Bone resorption is typically subperiosteal, subchondral, and subligamentous in distribution. Characteristic sites of subperiosteal resorption include the radial margins of the middle phalanges of the hands and the medial margins of the proximal tibiae. Characteristic sites of subchondral resorption include the sacroiliac joints, pubic symphysis, and acromioclavicular joints.
 Osteosclerosis and soft tissue and vascular calcifications are more common in patients with secondary hyperparathyroidism due to renal disease compared to patients with primary hyperparathyroidism.
Osteosclerosis and soft tissue and vascular calcifications are more common in patients with secondary hyperparathyroidism due to renal disease compared to patients with primary hyperparathyroidism.
 Osteosclerosis in these patients usually involves the axial skeleton, but may also involve long bones, pelvis, clavicles, and the bones of the face. In the spine, the bone subjacent to the cartilaginous endplate is usually preferentially involved, leading to the characteristic “rugger-jersey spine” appearance. Small subchondral resorptive changes may be seen at the discovertebral junctions.
Osteosclerosis in these patients usually involves the axial skeleton, but may also involve long bones, pelvis, clavicles, and the bones of the face. In the spine, the bone subjacent to the cartilaginous endplate is usually preferentially involved, leading to the characteristic “rugger-jersey spine” appearance. Small subchondral resorptive changes may be seen at the discovertebral junctions.
 Brown tumors or osteoclastomas are well-defined lytic lesions that may heal with sclerosis after treatment of the hyperparathyroidism.
Brown tumors or osteoclastomas are well-defined lytic lesions that may heal with sclerosis after treatment of the hyperparathyroidism.
 The skeletal changes associated with renal osteodys-trophy can be halted or reversed by early detection and careful management of vitamin D, parathyroid hormone, and mineral levels.
The skeletal changes associated with renal osteodys-trophy can be halted or reversed by early detection and careful management of vitamin D, parathyroid hormone, and mineral levels.
SUGGESTED READING
Murphey MD, Sartoris DJ, Quale JL, et al. Musculoskeletal manifestations of chronic renal insufficiency. Radiographics 1993;13:357–379.
Resnick D. Diagnosis of Bone and Joint Disorders (3rd ed). Philadelphia, PA: W.B. Saunders Company, 1995:1902–1905, 2036–2061.
Slatopolsky E. The role of calcium, phosphorus and vitamin D metabolism in the development of secondary hyperparathyroidism. Nephrol Dial Transplant 1998;13(Suppl 3):3–8.
Sundaram M. Founders lecture 2007: metabolic bone disease: what has changed in 30 years? Skeletal Radiol 2009;38:841–853.
EMILY N.
VINSON
HISTORY
A 33-year-old man with 2 months of worsening left hip pain and tenderness.
 FIGURES 5-2A and 5-2B (A) Anteroposterior radiograph of the pelvis with (B) magnification of the left hip. The right hip is normal in appearance. There is joint space narrowing of the left hip, along with indistinctness of the articular cortex of the left acetabulum and left femoral head. The supraacetabular line is not visualized on the left, a striking difference compared with the normal right hip. There is juxta-articular osteopenia with ill-defined periarticular lucencies and erosive changes.
FIGURES 5-2A and 5-2B (A) Anteroposterior radiograph of the pelvis with (B) magnification of the left hip. The right hip is normal in appearance. There is joint space narrowing of the left hip, along with indistinctness of the articular cortex of the left acetabulum and left femoral head. The supraacetabular line is not visualized on the left, a striking difference compared with the normal right hip. There is juxta-articular osteopenia with ill-defined periarticular lucencies and erosive changes.
DIFFERENTIAL DIAGNOSIS
 Septic joint: The presence of joint space narrowing, erosions, and juxta-articular osteopenia in the setting of a painful joint are very worrisome for a septic joint. While patients usually present within 2 weeks of symptom onset, the presentation may be delayed in the setting of low-virulence and mycobacterial organisms. Based on the clinical presentation and radiographic findings, this is the most likely diagnosis, and must be excluded prior to entertaining other possible diagnoses. When septic joint is a possible diagnosis, urgent arthro-centesis (joint aspiration) is indicated.
Septic joint: The presence of joint space narrowing, erosions, and juxta-articular osteopenia in the setting of a painful joint are very worrisome for a septic joint. While patients usually present within 2 weeks of symptom onset, the presentation may be delayed in the setting of low-virulence and mycobacterial organisms. Based on the clinical presentation and radiographic findings, this is the most likely diagnosis, and must be excluded prior to entertaining other possible diagnoses. When septic joint is a possible diagnosis, urgent arthro-centesis (joint aspiration) is indicated.
 Rheumatoid arthritis: The appearance of the left hip, with juxta-articular osteopenia, erosive changes, joint space narrowing, and the absence of bone formation such as osteophytosis are features suggestive of rheumatoid arthritis. However, rheumatoid arthritis is typically bilateral and symmetrical in distribution; in this case, the right hip is asymptomatic and normal in radiographic appearance, and therefore this is not the most likely diagnosis.
Rheumatoid arthritis: The appearance of the left hip, with juxta-articular osteopenia, erosive changes, joint space narrowing, and the absence of bone formation such as osteophytosis are features suggestive of rheumatoid arthritis. However, rheumatoid arthritis is typically bilateral and symmetrical in distribution; in this case, the right hip is asymptomatic and normal in radiographic appearance, and therefore this is not the most likely diagnosis.
 Psoriatic arthritis: It is unusual for the hip to be affected with psoriatic arthritis, and when it is, findings are often bilateral. In contrast to the current case, there is usually maintenance of juxta-articular bone mineralizaion and there are often peri articular bony proliferative changes. For these reasons, this is not the most likely diagnosis.
Psoriatic arthritis: It is unusual for the hip to be affected with psoriatic arthritis, and when it is, findings are often bilateral. In contrast to the current case, there is usually maintenance of juxta-articular bone mineralizaion and there are often peri articular bony proliferative changes. For these reasons, this is not the most likely diagnosis.
 Avascular necrosis (AVN) of the femoral head: AVN of the femoral head can cause areas of lucency and sclerosis in the femoral head, as are seen in this case. However, juxta-articular osteopenia and loss of visualization of the articular cortex are not features. Though joint space narrowing can occur due to secondary degenerative changes caused by AVN, it would be accompanied by osteophytosis and subchondral sclerosis, which are absent in this case. For these reasons, this is not the correct diagnosis.
Avascular necrosis (AVN) of the femoral head: AVN of the femoral head can cause areas of lucency and sclerosis in the femoral head, as are seen in this case. However, juxta-articular osteopenia and loss of visualization of the articular cortex are not features. Though joint space narrowing can occur due to secondary degenerative changes caused by AVN, it would be accompanied by osteophytosis and subchondral sclerosis, which are absent in this case. For these reasons, this is not the correct diagnosis.
 Calcium pyrophosphate deposition disease (CPPD): CPPD is a common cause of monoarticular arthritis in adult patients and an acute episode can be accompanied by a fever; however, CPPD usually affects patients over the age of 40 years. CPPD usually has an appearance similar to osteoarthritis; in the hip osteophytosis and subchondral sclerosis would be expected, and are absent in this case. In addition, there is no evidence of chondro-calcinosis of the visualized cartilaginous structures such as the pubic symphysis fibrocartilage in this case.
Calcium pyrophosphate deposition disease (CPPD): CPPD is a common cause of monoarticular arthritis in adult patients and an acute episode can be accompanied by a fever; however, CPPD usually affects patients over the age of 40 years. CPPD usually has an appearance similar to osteoarthritis; in the hip osteophytosis and subchondral sclerosis would be expected, and are absent in this case. In addition, there is no evidence of chondro-calcinosis of the visualized cartilaginous structures such as the pubic symphysis fibrocartilage in this case.
DIAGNOSIS
Septic joint. In this particular case, the organism isolated on culture of synovial fluid was Mycobacterium tuberculosis
KEY FACTS
Clinical
 An adult patient presenting with an acutely painful joint is a common medical emergency with many possible diagnoses, the most serious of which is a septic joint.
An adult patient presenting with an acutely painful joint is a common medical emergency with many possible diagnoses, the most serious of which is a septic joint.
 The most common presentation is that of a hot, swollen, tender joint with reduced range of motion. Patients usually present within 2 weeks of symptom onset, but presentation may be delayed in the setting of low-virulence organisms, fungal organisms, mycobacterial organisms, or infections of prosthetic joints. Fever is present in only about 60% of patients at the time of presentation.
The most common presentation is that of a hot, swollen, tender joint with reduced range of motion. Patients usually present within 2 weeks of symptom onset, but presentation may be delayed in the setting of low-virulence organisms, fungal organisms, mycobacterial organisms, or infections of prosthetic joints. Fever is present in only about 60% of patients at the time of presentation.
 Risk factors include underlying joint pathology (such as due to rheumatoid arthritis or osteoarthritis), age >80 years, immunocompromised status, prior intra-articular steroid injection, history of intravenous drug abuse, alcoholism, diabetes mellitus, the presence of cutaneous ulcers, recent joint surgery, and the presence of a joint prosthesis. However, these risk factors are only helpful when present; the absence of risk factors does not substantially reduce the likelihood of septic joint.
Risk factors include underlying joint pathology (such as due to rheumatoid arthritis or osteoarthritis), age >80 years, immunocompromised status, prior intra-articular steroid injection, history of intravenous drug abuse, alcoholism, diabetes mellitus, the presence of cutaneous ulcers, recent joint surgery, and the presence of a joint prosthesis. However, these risk factors are only helpful when present; the absence of risk factors does not substantially reduce the likelihood of septic joint.
 The most commonly involved joints are the knee, hip, ankle, wrist, shoulder, and elbow. Polyarticular involvement is rare but does occur.
The most commonly involved joints are the knee, hip, ankle, wrist, shoulder, and elbow. Polyarticular involvement is rare but does occur.
 The most common organisms isolated from patients with septic arthritis are staphylococci and streptococci. Infection is introduced into the joint by either hematog-enous spread (which is the most common route), direct inoculation due to penetrating trauma or iatrogenic procedure, or direct spread from an adjacent infection such as cellulitis or osteomyelitis.
The most common organisms isolated from patients with septic arthritis are staphylococci and streptococci. Infection is introduced into the joint by either hematog-enous spread (which is the most common route), direct inoculation due to penetrating trauma or iatrogenic procedure, or direct spread from an adjacent infection such as cellulitis or osteomyelitis.
 While adjunctive tests such as erythrocyte sedimentation rate, C-reactive protein, and white blood cell count are helpful in assessing a patient’s likelihood of having a septic joint and in monitoring a patient’s progress if septic joint is diagnosed, no clinical, laboratory, or radiographic findings are sufficiently sensitive and specific to exclude a septic joint.
While adjunctive tests such as erythrocyte sedimentation rate, C-reactive protein, and white blood cell count are helpful in assessing a patient’s likelihood of having a septic joint and in monitoring a patient’s progress if septic joint is diagnosed, no clinical, laboratory, or radiographic findings are sufficiently sensitive and specific to exclude a septic joint.
 When septic joint is a possibility, urgent arthrocentesis is indicated. However, Gram stain and culture of synovial fluid, obtained prior to antibiotic administration, identify the causative organism in only approximately 67% of cases of septic joint; thus, even a negative culture does not exclude a septic joint, though it does make the diagnosis less likely. An elevated synovial fluid white cell count increases the likelihood of septic arthritis, but is also not sufficiently reliable to confirm or exclude infection.
When septic joint is a possibility, urgent arthrocentesis is indicated. However, Gram stain and culture of synovial fluid, obtained prior to antibiotic administration, identify the causative organism in only approximately 67% of cases of septic joint; thus, even a negative culture does not exclude a septic joint, though it does make the diagnosis less likely. An elevated synovial fluid white cell count increases the likelihood of septic arthritis, but is also not sufficiently reliable to confirm or exclude infection.
 Blood cultures should also be drawn if septic joint is suspected, and in some patients positive blood cultures will provide organism identification even when synovial fluid cultures are negative.
Blood cultures should also be drawn if septic joint is suspected, and in some patients positive blood cultures will provide organism identification even when synovial fluid cultures are negative.
 Treatment includes appropriate antibiotic coverage, removal of purulent material from the joint space, and supportive measures.
Treatment includes appropriate antibiotic coverage, removal of purulent material from the joint space, and supportive measures.
 Delayed or inadequate treatment for septic joint can lead to permanent joint damage and functional disability due to rapid, irreversible cartilage destruction. Even in the setting of prompt and appropriate treatment, sepsis and/or permanent joint damage may occur. In addition, there is significant mortality, with fatality rates estimated at 7% to 15% despite the use of antibiotics.
Delayed or inadequate treatment for septic joint can lead to permanent joint damage and functional disability due to rapid, irreversible cartilage destruction. Even in the setting of prompt and appropriate treatment, sepsis and/or permanent joint damage may occur. In addition, there is significant mortality, with fatality rates estimated at 7% to 15% despite the use of antibiotics.
Radiologic
 Imaging techniques cannot accurately distinguish infective from noninfective inflammatory arthritis. In addition, radiographs of the affected joint may be normal, especially early in the course of infection. Urgent arthrocentesis is indicated if septic joint is clinically suspected, even in the absence of radiographic findings.
Imaging techniques cannot accurately distinguish infective from noninfective inflammatory arthritis. In addition, radiographs of the affected joint may be normal, especially early in the course of infection. Urgent arthrocentesis is indicated if septic joint is clinically suspected, even in the absence of radiographic findings.
 When radiographs are abnormal, findings that may be seen include soft tissue swelling, joint effusion (which is particularly visible on radiographs of the elbow, knee, and ankle), uniform joint space narrowing due to cartilage destruction, juxta-articular osteopenia, and erosions. MR findings that may be seen in the setting of septic joint include joint effusion, surrounding soft tissue edema, diffuse joint space narrowing, cartilage loss, adjacent bone marrow edema, and erosions.
When radiographs are abnormal, findings that may be seen include soft tissue swelling, joint effusion (which is particularly visible on radiographs of the elbow, knee, and ankle), uniform joint space narrowing due to cartilage destruction, juxta-articular osteopenia, and erosions. MR findings that may be seen in the setting of septic joint include joint effusion, surrounding soft tissue edema, diffuse joint space narrowing, cartilage loss, adjacent bone marrow edema, and erosions.
 MR imaging can be useful in delineating the extent of infection, identifying coexistent osteomyelitis, and identifying the extension of infected material into the periarticular soft tissues. However, MR imaging is not sufficiently sensitive or specific in diagnosing septic arthritis to be diagnostically useful. Therefore, arthrocentesis should not be delayed for the sake of advanced imaging.
MR imaging can be useful in delineating the extent of infection, identifying coexistent osteomyelitis, and identifying the extension of infected material into the periarticular soft tissues. However, MR imaging is not sufficiently sensitive or specific in diagnosing septic arthritis to be diagnostically useful. Therefore, arthrocentesis should not be delayed for the sake of advanced imaging.
 For most joints, arthrocentesis is performed under the guidance of fluoroscopy. For some joints, such as the sternoclavicular or sacroiliac joints, CT guidance is often favored. Pediatric hip aspirations are commonly performed utilizing ultrasound guidance.
For most joints, arthrocentesis is performed under the guidance of fluoroscopy. For some joints, such as the sternoclavicular or sacroiliac joints, CT guidance is often favored. Pediatric hip aspirations are commonly performed utilizing ultrasound guidance.
 Arthrography (the instillation of contrast material) during arthrocentesis is necessary to confirm the intra-articular location of the needle. In addition, arthrography may reveal the presence of sinus tracts.
Arthrography (the instillation of contrast material) during arthrocentesis is necessary to confirm the intra-articular location of the needle. In addition, arthrography may reveal the presence of sinus tracts.
 Needle approaches during arthrocentesis should avoid overlying skin ulcers, areas of inflammation/ cellulitis, skin lesions such as psoriasis, and visualized sinus tracts if possible to avoid inoculating a sterile joint during the procedure. If cross-sectional imaging is available for review prior to the procedure, overlying fluid collections/ abscesses should be noted and aspirated separately, and not traversed by the needle on the way into the joint.
Needle approaches during arthrocentesis should avoid overlying skin ulcers, areas of inflammation/ cellulitis, skin lesions such as psoriasis, and visualized sinus tracts if possible to avoid inoculating a sterile joint during the procedure. If cross-sectional imaging is available for review prior to the procedure, overlying fluid collections/ abscesses should be noted and aspirated separately, and not traversed by the needle on the way into the joint.
 Synovial fluid is commonly sent to the laboratory for cell count and differential, Gram stain, and aerobic and anaerobic cultures. Other stains and cultures, including fungal and acid-fast bacillus (AFB), are sent when atypical organisms are suspected. The fluid may also be examined for the presence of crystals if gout or CPPD are diagnostic possibilities.
Synovial fluid is commonly sent to the laboratory for cell count and differential, Gram stain, and aerobic and anaerobic cultures. Other stains and cultures, including fungal and acid-fast bacillus (AFB), are sent when atypical organisms are suspected. The fluid may also be examined for the presence of crystals if gout or CPPD are diagnostic possibilities.
 If the aspirate contains pus, as much as possible should be removed at the time of arthrocentesis.
If the aspirate contains pus, as much as possible should be removed at the time of arthrocentesis.
 If no or insufficient fluid is retrieved during arthrocentesis (a “dry tap”), the needle should be repositioned several times; arthrography should be performed to confirm intra-articular position of the needle and to look for the presence of a sinus tract which may have a decompressing effect on the joint, making retrieval of fluid difficult. In the setting of a dry tap, some practitioners routinely perform joint washings with nonbacteriostatic sterile saline and send that material for culture, though the added diagnostic utility of this practice is unknown.
If no or insufficient fluid is retrieved during arthrocentesis (a “dry tap”), the needle should be repositioned several times; arthrography should be performed to confirm intra-articular position of the needle and to look for the presence of a sinus tract which may have a decompressing effect on the joint, making retrieval of fluid difficult. In the setting of a dry tap, some practitioners routinely perform joint washings with nonbacteriostatic sterile saline and send that material for culture, though the added diagnostic utility of this practice is unknown.
SUGGESTED READING
Lin HM, Learch TJ, White EA, Gottsegen CJ. Emergency joint aspiration: a guide for radiologists on call. Radiographics 2009;29:1139–1158.
Margaretten ME, Kohlwes J, Moore D, Bent S. Does this adult patient have septic arthritis? JAMA 2007;297:1478–1488.
Mathews CJ, Kingsley G, Field M, et al. Management of septic arthritis: a systematic review. Ann Rheum Dis 2007;66:440–445.
Mathews CJ, Weston VC, Jones A, et al. Bacterial septic arthritis in adults. Lancet 2010;375:846–855.
CHARLES E.
SPRITZER
HISTORY
A 39-year-old man with pain, tenderness, and mass-like swelling of the medial aspect of the left knee.
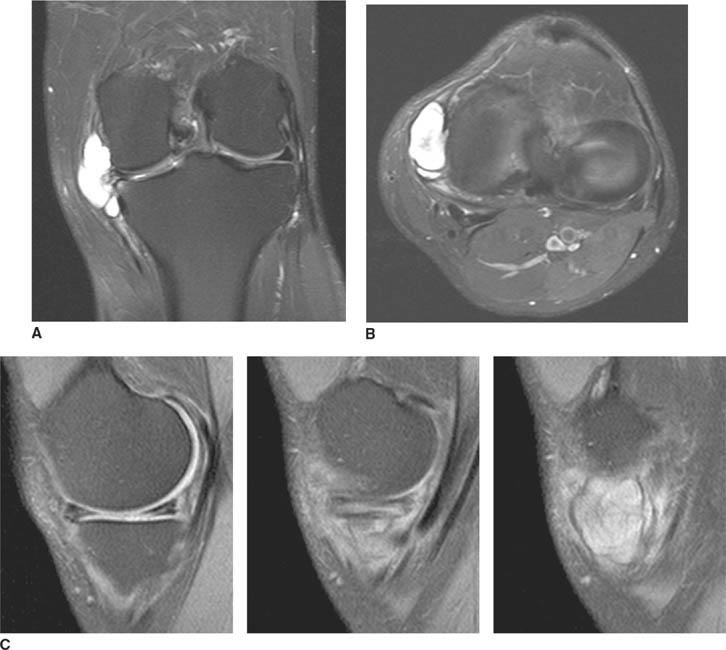
 FIGURES 5-3A, 5-3B and 5-3C (A) Coronal and (B) axial fat suppressed fast spin echo T2-weighted MR images of the knee. (C) Sagittal fat suppressed proton density-weighted MR images of the medial knee. A loculated fluid signal intensity collection is seen adjacent to the medial joint line on T2-weighted images (A and B). There is diffuse intermediate intensity signal abnormality within the substance of the periphery of the medial meniscus body (C), and this intrameniscal signal abnormality appears to extend directly into the adjacent fluid signal intensity collection in the parameniscal soft tissues (A and C). There is signal abnormality within the medial meniscus which extends to the articular surface of the medial meniscus (A and C), with oblique undersurface and horizontal components.
FIGURES 5-3A, 5-3B and 5-3C (A) Coronal and (B) axial fat suppressed fast spin echo T2-weighted MR images of the knee. (C) Sagittal fat suppressed proton density-weighted MR images of the medial knee. A loculated fluid signal intensity collection is seen adjacent to the medial joint line on T2-weighted images (A and B). There is diffuse intermediate intensity signal abnormality within the substance of the periphery of the medial meniscus body (C), and this intrameniscal signal abnormality appears to extend directly into the adjacent fluid signal intensity collection in the parameniscal soft tissues (A and C). There is signal abnormality within the medial meniscus which extends to the articular surface of the medial meniscus (A and C), with oblique undersurface and horizontal components.
DIFFERENTIAL DIAGNOSIS
 Popliteal cyst: These cysts tend to be located more posteriorly and not as medially as in this case. The neck of a popliteal cyst protrudes through the space between the medial head of the gastrocnemius and the semimembranosus muscles, not through the medial aspect of the joint as in this case.
Popliteal cyst: These cysts tend to be located more posteriorly and not as medially as in this case. The neck of a popliteal cyst protrudes through the space between the medial head of the gastrocnemius and the semimembranosus muscles, not through the medial aspect of the joint as in this case.
 Parameniscal cyst and tear of the medial meniscus: There is signal abnormality in the medial meniscus which extends to the articular surface, diagnostic of a menis-cal tear. A cystic structure emanating from an abnormal appearing meniscus, which in this case is the torn medial meniscus, is diagnostic of a parameniscal cyst.
Parameniscal cyst and tear of the medial meniscus: There is signal abnormality in the medial meniscus which extends to the articular surface, diagnostic of a menis-cal tear. A cystic structure emanating from an abnormal appearing meniscus, which in this case is the torn medial meniscus, is diagnostic of a parameniscal cyst.
 Ganglion cyst or synovial cyst: Because it is difficult to tell based on imaging features whether or not a synovial lining is truly present, the terms “ganglion cyst” and “synovial cyst” are sometimes used interchangeably in imaging. While the signal intensity of the loculated collection is consistent with a fluid-filled/jellylike viscous substance, and while this collection is technically a “ganglion” in the most general sense, the fact that this collection emanates from the medial meniscus distinguishes this entity from a simple “ganglion.” This distinction is important in the management of these lesions, which differs from that of simple ganglia as the underlying meniscal abnormality must be addressed.
Ganglion cyst or synovial cyst: Because it is difficult to tell based on imaging features whether or not a synovial lining is truly present, the terms “ganglion cyst” and “synovial cyst” are sometimes used interchangeably in imaging. While the signal intensity of the loculated collection is consistent with a fluid-filled/jellylike viscous substance, and while this collection is technically a “ganglion” in the most general sense, the fact that this collection emanates from the medial meniscus distinguishes this entity from a simple “ganglion.” This distinction is important in the management of these lesions, which differs from that of simple ganglia as the underlying meniscal abnormality must be addressed.
 Pes anserinus bursitis: Fluid and/or edema are often seen in association with inflammation of the pes anserine, namely, the distal semitendinosis, sartorius, and gracilis tendons. Thus, fluid collections due to pes bur-sitis are medial in location, like the current case, but are typically inferior to the joint line, and therefore this is not the correct diagnosis.
Pes anserinus bursitis: Fluid and/or edema are often seen in association with inflammation of the pes anserine, namely, the distal semitendinosis, sartorius, and gracilis tendons. Thus, fluid collections due to pes bur-sitis are medial in location, like the current case, but are typically inferior to the joint line, and therefore this is not the correct diagnosis.
 Medial collateral ligament bursitis: A fluid-filled medial or tibial collateral ligament bursa is located medially, at the level of the joint line, and deep to the medial collateral ligament, features that make this a possible diagnosis in this case. However, in this case the fluid is seen to emanate from a region of signal abnormality in the medial meniscus, and therefore this is not the correct diagnosis.
Medial collateral ligament bursitis: A fluid-filled medial or tibial collateral ligament bursa is located medially, at the level of the joint line, and deep to the medial collateral ligament, features that make this a possible diagnosis in this case. However, in this case the fluid is seen to emanate from a region of signal abnormality in the medial meniscus, and therefore this is not the correct diagnosis.
DIAGNOSIS
Parameniscal cyst and tear of the medial meniscus
KEY FACTS
Clinical
 Classically, these lesions have been referred to as “meniscal cysts.” With the advent of advanced imaging has come the recognition that meniscal cysts may exist within the confines of the meniscus (some refer to this as an “intrameniscal cyst”) with or without extension into the parameniscal soft tissues. Now, some describe the parameniscal cystic component of these lesions as a “parameniscal cyst” or as a “meniscal cyst with a parameniscal component.” Some continue to use the more general term “meniscal cyst” to refer to the entire spectrum of cystic lesions of the meniscus.
Classically, these lesions have been referred to as “meniscal cysts.” With the advent of advanced imaging has come the recognition that meniscal cysts may exist within the confines of the meniscus (some refer to this as an “intrameniscal cyst”) with or without extension into the parameniscal soft tissues. Now, some describe the parameniscal cystic component of these lesions as a “parameniscal cyst” or as a “meniscal cyst with a parameniscal component.” Some continue to use the more general term “meniscal cyst” to refer to the entire spectrum of cystic lesions of the meniscus.
 Meniscal cysts contain jelly-like mucinous or synovial fluid. They may occur laterally or medially, and most but not all meniscal cysts are seen in association with a meniscal tear extending to the articular surface of the meniscus.
Meniscal cysts contain jelly-like mucinous or synovial fluid. They may occur laterally or medially, and most but not all meniscal cysts are seen in association with a meniscal tear extending to the articular surface of the meniscus.
 The pathogenesis and treatment of meniscal cysts is somewhat controversial. Possible etiologies include traumatic and degenerative.
The pathogenesis and treatment of meniscal cysts is somewhat controversial. Possible etiologies include traumatic and degenerative.
 Some advocate a “one-way valve” mechanism, whereby synovial fluid is absorbed via a meniscal tear into the substance of the meniscus, where it collects and may subsequently spread into the parameniscal soft tissues. Others favor that these cysts—particularly when no meniscal tear is evident—form from a myxoid degenerative process within the substance of the meniscus, which may enlarge the meniscus (“intrameniscal cyst”) and which may subsequently be expressed into the adjacent soft tissues, forming a parameniscal component or “parameniscal cyst.”
Some advocate a “one-way valve” mechanism, whereby synovial fluid is absorbed via a meniscal tear into the substance of the meniscus, where it collects and may subsequently spread into the parameniscal soft tissues. Others favor that these cysts—particularly when no meniscal tear is evident—form from a myxoid degenerative process within the substance of the meniscus, which may enlarge the meniscus (“intrameniscal cyst”) and which may subsequently be expressed into the adjacent soft tissues, forming a parameniscal component or “parameniscal cyst.”
 For most ganglion cysts, excision of the lesion is sufficient. However, if a parameniscal cyst is excised in a similar fashion, it will typically recur. As such, the diagnosis of a meniscal cyst in this situation requires treatment of the underlying meniscal abnormality to prevent recurrence.
For most ganglion cysts, excision of the lesion is sufficient. However, if a parameniscal cyst is excised in a similar fashion, it will typically recur. As such, the diagnosis of a meniscal cyst in this situation requires treatment of the underlying meniscal abnormality to prevent recurrence.
 In the past, treatment of meniscal cysts often consisted of open arthrotomy and complete meniscectomy. Increased appreciation of the importance of the conservation of meniscal tissue has led to the arthroscopic treatment of meniscal cysts with either open excision of any parameniscal cystic component and arthroscopic assessment of the meniscus involved, or a entirely arthroscopic procedure with arthroscopic decompression of the cyst prior to addressing the meniscus itself. In either case, a careful arthroscopic assessment of the meniscus involved is warranted to inspect for and address any meniscal tear to the articular surface that may be present.
In the past, treatment of meniscal cysts often consisted of open arthrotomy and complete meniscectomy. Increased appreciation of the importance of the conservation of meniscal tissue has led to the arthroscopic treatment of meniscal cysts with either open excision of any parameniscal cystic component and arthroscopic assessment of the meniscus involved, or a entirely arthroscopic procedure with arthroscopic decompression of the cyst prior to addressing the meniscus itself. In either case, a careful arthroscopic assessment of the meniscus involved is warranted to inspect for and address any meniscal tear to the articular surface that may be present.
Radiologic
 MR imaging of the knee is useful in distinguishing meniscal cysts from other cystic lesions around the knee, such as bursae, which may extend adjacent to and even abut the outer edge of the meniscus; a true meniscal cyst has a clear, identifiable connection of the parameniscal cystic component to the adjacent meniscus, and the demonstration of abnormal signal intensity within the adjacent meniscus itself. This is particularly important medially, where several bursae are located in the soft tissues adjacent to the medial meniscus, and can sometimes mimic parameniscal cysts.
MR imaging of the knee is useful in distinguishing meniscal cysts from other cystic lesions around the knee, such as bursae, which may extend adjacent to and even abut the outer edge of the meniscus; a true meniscal cyst has a clear, identifiable connection of the parameniscal cystic component to the adjacent meniscus, and the demonstration of abnormal signal intensity within the adjacent meniscus itself. This is particularly important medially, where several bursae are located in the soft tissues adjacent to the medial meniscus, and can sometimes mimic parameniscal cysts.
 On MR imaging, an intrameniscal cyst demonstrates abnormal signal intensity, usually of intermediate signal intensity, within the substance of the meniscus with associated bulging, convex borders. The convex contour of the meniscus may not be evident in cases with associated meniscal tears and/or parameniscal cystic components due to decompression of the meniscus itself.
On MR imaging, an intrameniscal cyst demonstrates abnormal signal intensity, usually of intermediate signal intensity, within the substance of the meniscus with associated bulging, convex borders. The convex contour of the meniscus may not be evident in cases with associated meniscal tears and/or parameniscal cystic components due to decompression of the meniscus itself.
 When a parameniscal cystic component is present, it is almost always of fluid signal intensity on T2-weighted MR images, and usually manifests as a loculated fluid collection extending from the joint line.
When a parameniscal cystic component is present, it is almost always of fluid signal intensity on T2-weighted MR images, and usually manifests as a loculated fluid collection extending from the joint line.
 MR imaging is useful in determining whether or not an associated meniscal tear is present, which is an important consideration in planning treatment. Meniscus sensitive sequences, such as proton density or Tl-weighted MR imaging, are utilized to inspect for associated tears. These tears often have a horizontal component as well as an oblique component to the articular surface.
MR imaging is useful in determining whether or not an associated meniscal tear is present, which is an important consideration in planning treatment. Meniscus sensitive sequences, such as proton density or Tl-weighted MR imaging, are utilized to inspect for associated tears. These tears often have a horizontal component as well as an oblique component to the articular surface.
SUGGESTED READING
Anderson JJ, Connor GF, Helms CA. New observations on meniscal cysts. Skeletal Radiol 2010;39:1187–1191.
Campbell SE, Sanders TG, Morrison WB. MR imaging of meniscal cysts: incidence, location, and clinical significance. Am J Roentgenol 2001;177:409–413.
De Maeseneer M, Shahabpour M, Vanderdood K, et al. MR imaging of meniscal cysts: evaluation of location and extension using a three-layer approach. Eur J Radiol 2001;39:117–124.
Sarimo J, Rainio P, Rantanen J, Orava S. Comparison of two procedures for meniscal cysts: a report of 35 patients with a mean follow-up of 33 months. Am J Sports Med 2002;30:704–707.
Tschirch FTC, Schmid MR, Pfirrmann CWA, et al. Prevalence and size of meniscal cysts, ganglionic cysts, synovial cysts of the popliteal space, fluid-filled bursae, and other fluid collections in asymptomatic knees on MR imaging. Am J Roentgenol 2003;180:1431–1436.
ROBERT M.
VANDEMARK
AND
R. LEE
COTHRAN. JR.
HISTORY
Patient A: A 35-year-old man who fell from a ladder onto an outstretched hand and now complains of shoulder pain. Patient B: A 45-year-old woman who complains of severe shoulder pain following a grand mal seizure.
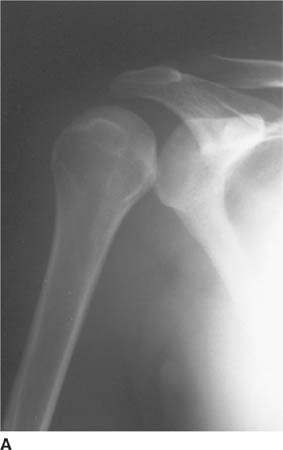
 FIGURE 5-4A Anteroposterior internal rotation radiograph of the shoulder in patient A. There is widening of the joint space.
FIGURE 5-4A Anteroposterior internal rotation radiograph of the shoulder in patient A. There is widening of the joint space.
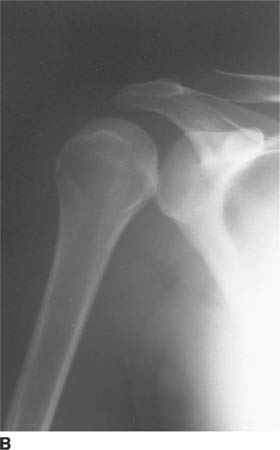
 FIGURE 5-4B Anteroposterior external rotation radiograph of the shoulder in patient A. This view also shows identical joint space widening, which indicates lack of any motion at the glenohu-meral joint.
FIGURE 5-4B Anteroposterior external rotation radiograph of the shoulder in patient A. This view also shows identical joint space widening, which indicates lack of any motion at the glenohu-meral joint.
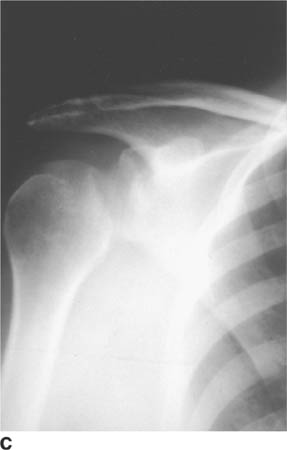
 FIGURE 5-4C Anteroposterior radiograph of the shoulder in patient B. There is widening of the joint space and an oblique sclerotic band through the articular segment of the humerus.
FIGURE 5-4C Anteroposterior radiograph of the shoulder in patient B. There is widening of the joint space and an oblique sclerotic band through the articular segment of the humerus.
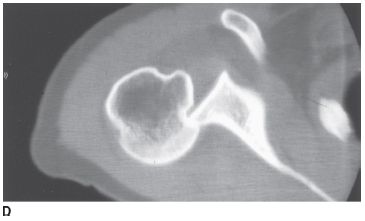
 FIGURE 5-4D Axial CT of the glenohumeral joint in patient B. There is an impaction fracture produced by collision of the posterior glenoid rim on the humeral head.
FIGURE 5-4D Axial CT of the glenohumeral joint in patient B. There is an impaction fracture produced by collision of the posterior glenoid rim on the humeral head.
DIFFERENTIAL DIAGNOSIS
 Anterior dislocation of the shoulder: Anterior shoulder dislocations characteristically cause anteroinferior displacement of the humeral head relative to the gle-noid. This position of the humeral head is never seen in patients with posterior dislocation. In addition, anterior dislocation leads to total obscuration of the normal glenohumeral joint space due to overlap of the humeral head and the glenoid. This is not the correct diagnosis based on the imaging appearance.
Anterior dislocation of the shoulder: Anterior shoulder dislocations characteristically cause anteroinferior displacement of the humeral head relative to the gle-noid. This position of the humeral head is never seen in patients with posterior dislocation. In addition, anterior dislocation leads to total obscuration of the normal glenohumeral joint space due to overlap of the humeral head and the glenoid. This is not the correct diagnosis based on the imaging appearance.
 Posterior dislocation of the shoulder: Posterior dislocations are rare but are characteristic in patients with seizures, electrical injury, and, occasionally, blunt injury to the anterior aspect of the shoulder. In virtually all cases of posterior dislocation, the humeral head and glenoid remain in the same transverse plane, unlike anterior dislocation, where the humeral head displaces inferomedially. Some posterior dislocations give the appearance of a widened glenohumeral joint space, caused by “perching” of the humeral head on the posterior glenoid. As a result, the distance between the humeral head and the anterior glenoid is widened, which gives the false impression of a widened joint space. This feature is never seen in anterior dislocation. This is the correct diagnosis.
Posterior dislocation of the shoulder: Posterior dislocations are rare but are characteristic in patients with seizures, electrical injury, and, occasionally, blunt injury to the anterior aspect of the shoulder. In virtually all cases of posterior dislocation, the humeral head and glenoid remain in the same transverse plane, unlike anterior dislocation, where the humeral head displaces inferomedially. Some posterior dislocations give the appearance of a widened glenohumeral joint space, caused by “perching” of the humeral head on the posterior glenoid. As a result, the distance between the humeral head and the anterior glenoid is widened, which gives the false impression of a widened joint space. This feature is never seen in anterior dislocation. This is the correct diagnosis.
 Pseudodislocation of the shoulder: This term applies to a clinical presentation in which physical findings suggest an abnormal position of the humeral head consistent with a shoulder dislocation. Subsequent radiographs fail to show a dislocation but do demonstrate inferior subluxation of the humeral head relative to the glenoid. Pseudodislocation can be seen in the setting of brachial plexus injury, chronic shoulder joint instability, hemarthrosis, and, occasionally, pyarthrosis. The current cases do not demonstrate inferior subluxation of the humeral head, and instead demonstrate a posterior position of the humeral head relative to the gle-noid; therefore, this is not the correct diagnosis.
Pseudodislocation of the shoulder: This term applies to a clinical presentation in which physical findings suggest an abnormal position of the humeral head consistent with a shoulder dislocation. Subsequent radiographs fail to show a dislocation but do demonstrate inferior subluxation of the humeral head relative to the glenoid. Pseudodislocation can be seen in the setting of brachial plexus injury, chronic shoulder joint instability, hemarthrosis, and, occasionally, pyarthrosis. The current cases do not demonstrate inferior subluxation of the humeral head, and instead demonstrate a posterior position of the humeral head relative to the gle-noid; therefore, this is not the correct diagnosis.
DIAGNOSIS
Posterior dislocation of the shoulder
KEY FACTS
Clinical
 Less than 3% of all shoulder dislocations are posterior.
Less than 3% of all shoulder dislocations are posterior.
 The characteristic setting for this injury is in a patient after a seizure.
The characteristic setting for this injury is in a patient after a seizure.
Radiologic
 “Fixed” internal rotation should immediately raise the possibility of posterior dislocation of the shoulder.
“Fixed” internal rotation should immediately raise the possibility of posterior dislocation of the shoulder.
 A “positive” rim sign is present when the distance between the anterior glenoid rim and the humerus is >6 mm.
A “positive” rim sign is present when the distance between the anterior glenoid rim and the humerus is >6 mm.
 Posterior dislocation remains a challenging diagnosis when the axillary or “Y” views are unavailable. Such would be the case on portable chest radiographs obtained for patients with blunt trauma, electrical injury, or seizure disorders. Under these circumstances, it is important to recognize the clues of posterior dislocation on an anteroposterior view alone. The radiologist must recognize fixed internal rotation, widening of the joint space (rim sign), and loss of the overlap appearance (half moon) of the normal shoulder joint as characteristic findings of posterior dislocation on a frontal film.
Posterior dislocation remains a challenging diagnosis when the axillary or “Y” views are unavailable. Such would be the case on portable chest radiographs obtained for patients with blunt trauma, electrical injury, or seizure disorders. Under these circumstances, it is important to recognize the clues of posterior dislocation on an anteroposterior view alone. The radiologist must recognize fixed internal rotation, widening of the joint space (rim sign), and loss of the overlap appearance (half moon) of the normal shoulder joint as characteristic findings of posterior dislocation on a frontal film.
 A posttraumatic dent in the humeral head (trough sign) is a helpful sign but is not always present. Posterior dislocations can also be seen in conjunction with comminuted fracture-dislocations of the proximal humerus. In any event, the reader should keep in mind that posterior dislocations when missed can easily escape clinical detection and become chronic dislocations. It is not unusual for patients with chronic posterior dislocation to remain undiagnosed for months at a time. The radiologist must always have a high index for a “missed” posterior dislocation when reviewing outpatient radiographs in patients with chronic shoulder pain.
A posttraumatic dent in the humeral head (trough sign) is a helpful sign but is not always present. Posterior dislocations can also be seen in conjunction with comminuted fracture-dislocations of the proximal humerus. In any event, the reader should keep in mind that posterior dislocations when missed can easily escape clinical detection and become chronic dislocations. It is not unusual for patients with chronic posterior dislocation to remain undiagnosed for months at a time. The radiologist must always have a high index for a “missed” posterior dislocation when reviewing outpatient radiographs in patients with chronic shoulder pain.
 A clinical history of any or all of the following symptoms—a frozen shoulder, limited range of motion, and old trauma—should motivate the radiologist to obtain a dislocation view (axillary view preferred) to exclude the possibility of a chronic posterior dislocation.
A clinical history of any or all of the following symptoms—a frozen shoulder, limited range of motion, and old trauma—should motivate the radiologist to obtain a dislocation view (axillary view preferred) to exclude the possibility of a chronic posterior dislocation.
SUGGESTED READING
Helms CA. Fundamentals of Skeletal Radiology (3rd ed). Philadelphia, PA: Elsevier Saunders, 2005:97–101.
Resnick D. Diagnosis of Bone and Joint Disorders (4th ed). Philadelphia, PA: Saunders, 2002:2784–2796.
Rogers LF, Lenchik L. The shoulder and humeral shaft. In LF Rogers (ed), Radiology of Skeletal Trauma. Philadelphia, PA: Saunders, 2002: 662–669.
EMILY N.
VINSON
HISTORY
A 57-year-old man who has had pain in his hands for over 25 years. On physical examination, there are patches of red, scaly skin behind his ears, on the left frontal portion of his scalp, and on his left elbow. In addition, several of his fingernails appear thickened and discolored.
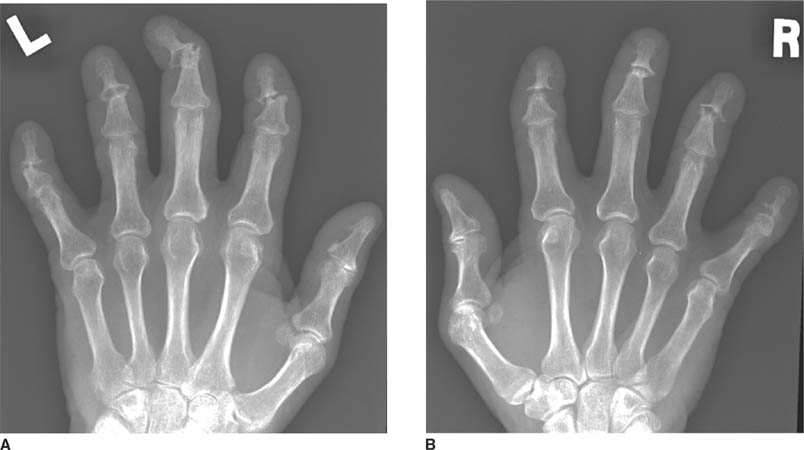
 FIGURES 5-5A and 5-5B Anteroposterior radiographs of the left (A) and right (B) hands demonstrate severe erosive changes involving the DIP joints bilaterally, with “pencil-in-cup” deformities. Bone mineralization is preserved, and the MCP joints are relatively spared.
FIGURES 5-5A and 5-5B Anteroposterior radiographs of the left (A) and right (B) hands demonstrate severe erosive changes involving the DIP joints bilaterally, with “pencil-in-cup” deformities. Bone mineralization is preserved, and the MCP joints are relatively spared.
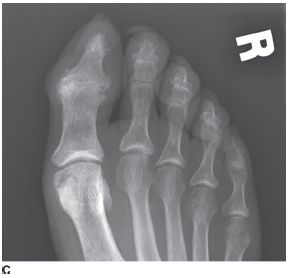
 FIGURE 5-5C Anteroposterior radiograph of the right foot toes. There is joint space loss of the great toe IP joint with periarticular erosions and new bone formation. There are similar changes at the second, third, and fourth DIP joints. There is erosion and sclerosis of the distal tuft of the great toe.
FIGURE 5-5C Anteroposterior radiograph of the right foot toes. There is joint space loss of the great toe IP joint with periarticular erosions and new bone formation. There are similar changes at the second, third, and fourth DIP joints. There is erosion and sclerosis of the distal tuft of the great toe.
DIFFERENTIAL DIAGNOSIS
 Rheumatoid arthritis (RA): RA has a predilection for bilateral symmetric involvement of the hands, as is seen in this case. However, RA tends to involve the more proximal joints of the hand and wrist such as the carpus, ulnar styloid, MCP joints, and PIP joints, whereas in this case involvement of the DIP joints predominates. The IP joint of the great toe can be involved in RA, but the more classic site for erosive change in the foot due to RA is the head of the fifth metatarsal, which does not appear involved in this case. There is no evidence of juxta-articular or generalized osteoporosis in this case, a common feature of RA. Perhaps most importantly, this case demonstrates bone proliferation, which should be absent in RA. For these reasons, this is not the correct diagnosis.
Rheumatoid arthritis (RA): RA has a predilection for bilateral symmetric involvement of the hands, as is seen in this case. However, RA tends to involve the more proximal joints of the hand and wrist such as the carpus, ulnar styloid, MCP joints, and PIP joints, whereas in this case involvement of the DIP joints predominates. The IP joint of the great toe can be involved in RA, but the more classic site for erosive change in the foot due to RA is the head of the fifth metatarsal, which does not appear involved in this case. There is no evidence of juxta-articular or generalized osteoporosis in this case, a common feature of RA. Perhaps most importantly, this case demonstrates bone proliferation, which should be absent in RA. For these reasons, this is not the correct diagnosis.
 Psoriatic arthritis: The severe erosive changes affecting the DIP joints of the hands with “pencil-in-cup” appearance and sparing of the MCP joints is most consistent with psoriatic arthritis. Other findings that support this diagnosis are proliferative erosive changes of the great toe IP joint, an “ivory phalanx” of the great toe distal phalanx, and maintenance of normal bone mineralization. The provided clinical history of skin lesions and nail involvement is also characteristic of this disorder.
Psoriatic arthritis: The severe erosive changes affecting the DIP joints of the hands with “pencil-in-cup” appearance and sparing of the MCP joints is most consistent with psoriatic arthritis. Other findings that support this diagnosis are proliferative erosive changes of the great toe IP joint, an “ivory phalanx” of the great toe distal phalanx, and maintenance of normal bone mineralization. The provided clinical history of skin lesions and nail involvement is also characteristic of this disorder.
 Reiter’s disease/reactive arthritis: Reactive arthritis has an identical radiologic appearance to psoriatic arthritis, including ill-defined erosions and bone proliferation. However, reactive arthritis has a slightly different distribution, with hand involvement very uncommon. In addition, the patient’s clinical history makes this diagnosis unlikely; reactive arthritis usually presents in males between the ages of 15 and 35 years and is often associated with urethritis and conjunctivitis rather than skin lesions. For these reasons, this is not the correct diagnosis.
Reiter’s disease/reactive arthritis: Reactive arthritis has an identical radiologic appearance to psoriatic arthritis, including ill-defined erosions and bone proliferation. However, reactive arthritis has a slightly different distribution, with hand involvement very uncommon. In addition, the patient’s clinical history makes this diagnosis unlikely; reactive arthritis usually presents in males between the ages of 15 and 35 years and is often associated with urethritis and conjunctivitis rather than skin lesions. For these reasons, this is not the correct diagnosis.
DIAGNOSIS
Psoriatic arthritis
KEY FACTS
Clinical
 Psoriatic arthritis is a chronic inflammatory arthritis associated with the skin disorder psoriasis, and is estimated to occur in up to 30% of patients with psoriasis.
Psoriatic arthritis is a chronic inflammatory arthritis associated with the skin disorder psoriasis, and is estimated to occur in up to 30% of patients with psoriasis.
 In the past, psoriatic arthritis was considered a “rheumatoid variant.” Psoriatic arthritis was recognized as an entity distinct from RA in the 1940s, following the discovery of the rheumatoid factor. Patients with pso-riatic arthritis are usually seronegative (meaning no rheumatoid factor is detected in their serum).
In the past, psoriatic arthritis was considered a “rheumatoid variant.” Psoriatic arthritis was recognized as an entity distinct from RA in the 1940s, following the discovery of the rheumatoid factor. Patients with pso-riatic arthritis are usually seronegative (meaning no rheumatoid factor is detected in their serum).
 Psoriatic arthritis is considered one of the spondyloar-thropathies due to the presence of spondylitis in up to 40% of patients and an association with HLA-B27 antigen.
Psoriatic arthritis is considered one of the spondyloar-thropathies due to the presence of spondylitis in up to 40% of patients and an association with HLA-B27 antigen.
 The skin manifestations of psoriasis usually precede the development of psoriatic arthritis by an average of 10 years. Less commonly, seen in 15% to 20% of patients, the arthritis will manifest earlier than evidence of psoriasis skin lesions.
The skin manifestations of psoriasis usually precede the development of psoriatic arthritis by an average of 10 years. Less commonly, seen in 15% to 20% of patients, the arthritis will manifest earlier than evidence of psoriasis skin lesions.
 Nail lesions are very common in patients with psoriatic arthritis (occurring in about 87%).
Nail lesions are very common in patients with psoriatic arthritis (occurring in about 87%).
 Up to 20% of patients with psoriatic arthritis will experience a very destructive, disabling form of arthritis.
Up to 20% of patients with psoriatic arthritis will experience a very destructive, disabling form of arthritis.
 Mild psoriatic arthritis may be managed with non-steroidal anti-inflammatory drugs. Newer treatment options for moderate to severe disease include disease-modifying antirheumatic drugs and biologic agents such as tumor necrosis factor-alpha inhibitors.
Mild psoriatic arthritis may be managed with non-steroidal anti-inflammatory drugs. Newer treatment options for moderate to severe disease include disease-modifying antirheumatic drugs and biologic agents such as tumor necrosis factor-alpha inhibitors.
Radiologic
 Psoriatic arthritis is an inflammatory arthritis characterized by uniform joint space narrowing, erosions, bone proliferation, periostitis, and enthesitis.
Psoriatic arthritis is an inflammatory arthritis characterized by uniform joint space narrowing, erosions, bone proliferation, periostitis, and enthesitis.
 Radiographic findings in psoriatic arthritis may be bilateral or unilateral, symmetric or asymmetric.
Radiographic findings in psoriatic arthritis may be bilateral or unilateral, symmetric or asymmetric.
 The most common site of involvement is the hands, and there are several different patterns of hand involvement: DIP and PIP joint involvement with sparing of the MCP joints and carpus; involvement of all joints of one to three fingers, with sparing of the other fingers; and diffuse involvement with a distribution similar to RA, differing from RA in that there are usually bone prolif-erative changes and DIP involvement present.
The most common site of involvement is the hands, and there are several different patterns of hand involvement: DIP and PIP joint involvement with sparing of the MCP joints and carpus; involvement of all joints of one to three fingers, with sparing of the other fingers; and diffuse involvement with a distribution similar to RA, differing from RA in that there are usually bone prolif-erative changes and DIP involvement present.
 There is often soft tissue swelling affecting an entire digit in the hand or foot, known as the “sausage digit.”
There is often soft tissue swelling affecting an entire digit in the hand or foot, known as the “sausage digit.”
 Erosions start at the margins of the joint and progress to involve the central portion. Erosions may become so destructive that the joint appears widened. The ends of phalangeal bones may become pointed, with saucer-ization of the articulating bone, causing the “pencil-in-cup” appearance. Acro-osteolysis may occur. The characteristic erosive changes seen in psoriatic arthritis help distinguish it from another of the seronegative spondyloarthropathies, ankylosing spondylitis.
Erosions start at the margins of the joint and progress to involve the central portion. Erosions may become so destructive that the joint appears widened. The ends of phalangeal bones may become pointed, with saucer-ization of the articulating bone, causing the “pencil-in-cup” appearance. Acro-osteolysis may occur. The characteristic erosive changes seen in psoriatic arthritis help distinguish it from another of the seronegative spondyloarthropathies, ankylosing spondylitis.
 Even in the setting of severe erosive disease, normal bone mineralization is usually maintained. Other radiographic features that help distinguish psoriatic arthritis from RA are the presence of bone proliferation and a distribution that is often asymmetric.
Even in the setting of severe erosive disease, normal bone mineralization is usually maintained. Other radiographic features that help distinguish psoriatic arthritis from RA are the presence of bone proliferation and a distribution that is often asymmetric.
 Bone proliferation occurs adjacent to erosions (and is often initially irregular and ill-defined in appearance), along the diaphysis (periostitis), across joints (ankylo-sis, particularly common at the interphalangeal joints), and at tendinous and ligamentous insertion sites (enthesitis).
Bone proliferation occurs adjacent to erosions (and is often initially irregular and ill-defined in appearance), along the diaphysis (periostitis), across joints (ankylo-sis, particularly common at the interphalangeal joints), and at tendinous and ligamentous insertion sites (enthesitis).
 Findings in the foot are similar to those in the hand. Destruction of the great toe IP joint is more common in psoriatic arthritis than with any other arthritis.
Findings in the foot are similar to those in the hand. Destruction of the great toe IP joint is more common in psoriatic arthritis than with any other arthritis.
 An “ivory phalanx” may be seen in psoriatic arthritis, due to sclerosis and/or bone proliferation affecting a distal phalanx in the foot (most commonly, the great toe distal phalanx).
An “ivory phalanx” may be seen in psoriatic arthritis, due to sclerosis and/or bone proliferation affecting a distal phalanx in the foot (most commonly, the great toe distal phalanx).
 Other appendicular joints may be affected, including the knee, ankle, and shoulder. Involvement of the hip joint is uncommon, although enthesitis affecting the greater trochanter is common.
Other appendicular joints may be affected, including the knee, ankle, and shoulder. Involvement of the hip joint is uncommon, although enthesitis affecting the greater trochanter is common.
 Up to half of patients with psoriatic arthritis will have involvement of the synovial portion of the SI joints, which is usually asymmetric but can be symmetric, characterized by erosive and bulky proliferative changes. Anky-losis can occur. Ossification of the ligamentous portion of the SI joints may also occur.
Up to half of patients with psoriatic arthritis will have involvement of the synovial portion of the SI joints, which is usually asymmetric but can be symmetric, characterized by erosive and bulky proliferative changes. Anky-losis can occur. Ossification of the ligamentous portion of the SI joints may also occur.
 In the spine, large, bulky, often asymmetric paraverte-bral ossifications are characteristic. Except in the cervical spine, the facet joints are usually spared.
In the spine, large, bulky, often asymmetric paraverte-bral ossifications are characteristic. Except in the cervical spine, the facet joints are usually spared.
SUGGESTED READING
Brower AC, Flemming DJ. Arthritis in Black and White (2nd ed). Philadelphia, PA: W.B. Saunders Company, 1997.
Gladman DD, Antoni C, Mease P, et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005;64(Suppl Il):ii14–ii17.
Jacobson JA, Girish G, Jiang Y, Resnick D. Radiographic evaluation of arthritis: inflammatory conditions. Radiology 2008;248:378–389.
Spira D, Kotter I, Henes J, et al. MRl findings in psoriatic arthritis of the hands. Am J Roentgenol 2010;195:1187–1193.
ROBERT M.
VANDEMARK
AND
R. LEE
COTHRAN, Jr.
HISTORY
A 21-year-old man who was a restrained passenger in a head-on collision with another vehicle.
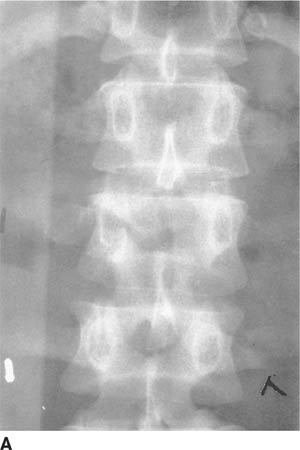
 FIGURE 5-6A Anteroposterior radiograph of the lumbar spine. There is a subtle horizontal lucency through the right pedicle of L2. In addition, there is distraction of the spinous processes of L1 and L2, resulting in a relative lucency of the L2 vertebral body.
FIGURE 5-6A Anteroposterior radiograph of the lumbar spine. There is a subtle horizontal lucency through the right pedicle of L2. In addition, there is distraction of the spinous processes of L1 and L2, resulting in a relative lucency of the L2 vertebral body.
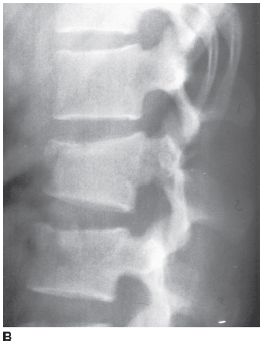
 FIGURE 5-6B Lateral radiograph of the lumbar spine. There is only a minor compression fracture of the body of L2.
FIGURE 5-6B Lateral radiograph of the lumbar spine. There is only a minor compression fracture of the body of L2.
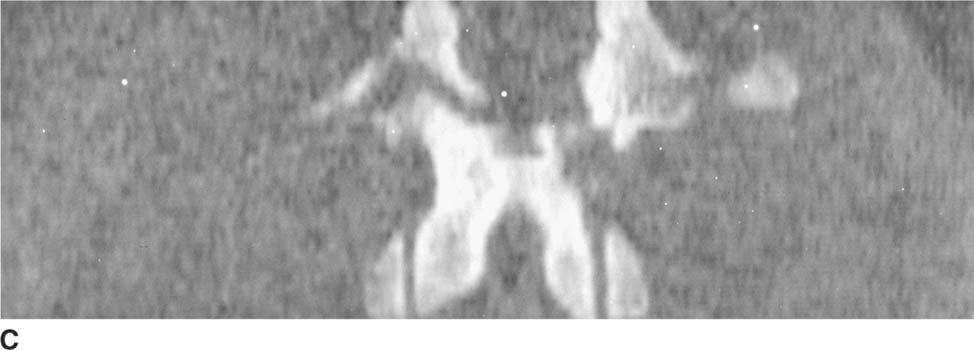
 FIGURE 5-6C Axial CT scan of the lumbar spine reformatted in the coronal plane. There is a horizontally oriented fracture of the posterior elements.
FIGURE 5-6C Axial CT scan of the lumbar spine reformatted in the coronal plane. There is a horizontally oriented fracture of the posterior elements.
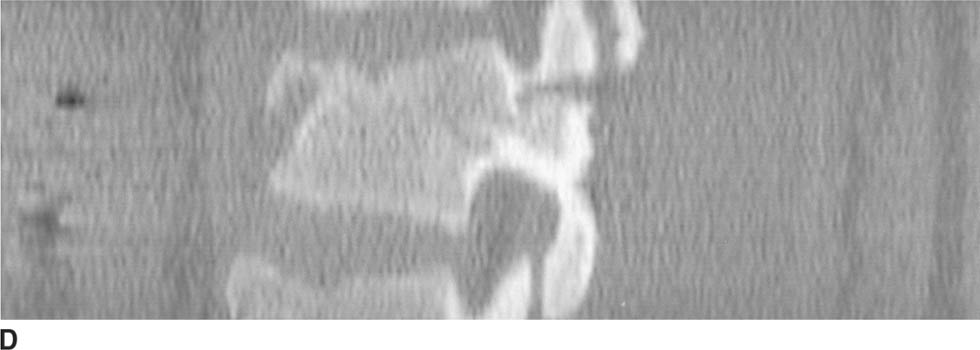
 FIGURE 5-6D Axial CT scan of the lumbar spine reformatted in the sagittal plane shows both the anterior and posterior components of the fracture.
FIGURE 5-6D Axial CT scan of the lumbar spine reformatted in the sagittal plane shows both the anterior and posterior components of the fracture.
DIFFERENTIAL DIAGNOSIS
 Simple compression fracture: Minor compression fractures of the vertebral column are common but do not usually disrupt the middle or posterior columns of stability. Usually the degree of compression is <25% of the vertebral body height, and there is no evidence of ret-ropulsion or extension of the fracture into the posterior elements. Given the posterior element involvement in this case, this is not the correct diagnosis.
Simple compression fracture: Minor compression fractures of the vertebral column are common but do not usually disrupt the middle or posterior columns of stability. Usually the degree of compression is <25% of the vertebral body height, and there is no evidence of ret-ropulsion or extension of the fracture into the posterior elements. Given the posterior element involvement in this case, this is not the correct diagnosis.
 Burst fracture: Burst fractures are caused by an axial load on the vertebral column as would occur during a fall from a significant height. CT examination would demonstrate radial dispersion of the vertebral body fragments with retropulsion into the spinal canal. The longitudinal distraction of the fractured posterior elements in this case is not consistent with a burst-type fracture; therefore, this is not the correct diagnosis.
Burst fracture: Burst fractures are caused by an axial load on the vertebral column as would occur during a fall from a significant height. CT examination would demonstrate radial dispersion of the vertebral body fragments with retropulsion into the spinal canal. The longitudinal distraction of the fractured posterior elements in this case is not consistent with a burst-type fracture; therefore, this is not the correct diagnosis.
 Chance fracture: The Chance fracture is produced by a flexion-distraction mechanism. Fracture lines are typically seen extending into the posterior elements of the vertebrae on a lateral view. Characteristically, subtle bilateral pedicle fractures can be seen on the AP radiograph and should be sought. Chance fractures sometimes produce only minimal anterior compression and can be confused with simple compression fractures when the pedicle and posterior element extension of the fracture are not appreciated. Based on the images provided, this is the correct diagnosis.
Chance fracture: The Chance fracture is produced by a flexion-distraction mechanism. Fracture lines are typically seen extending into the posterior elements of the vertebrae on a lateral view. Characteristically, subtle bilateral pedicle fractures can be seen on the AP radiograph and should be sought. Chance fractures sometimes produce only minimal anterior compression and can be confused with simple compression fractures when the pedicle and posterior element extension of the fracture are not appreciated. Based on the images provided, this is the correct diagnosis.
DIAGNOSIS
Chance fracture
KEY FACTS
Clinical
 Chance fractures are flexion-distraction injuries of the spine most commonly located in the thoracolumbar region and characterized by a transversely oriented facture with separation of the posterior elements in the longitudinal plane. These are unstable fractures with disruption of the middle and posterior columns, and often also involve extension into the anterior column.
Chance fractures are flexion-distraction injuries of the spine most commonly located in the thoracolumbar region and characterized by a transversely oriented facture with separation of the posterior elements in the longitudinal plane. These are unstable fractures with disruption of the middle and posterior columns, and often also involve extension into the anterior column.
 They are associated with seatbehVlapbelt restraint.
They are associated with seatbehVlapbelt restraint.
 There is a high association with intra-abdominal injuries.
There is a high association with intra-abdominal injuries.
 Despite the substantial posterior element injury seen in Chance fractures, neurologic deficits are less common than with other serious thoracolumbar spine injuries such as burst fractures or fracture-dislocations of the spine.
Despite the substantial posterior element injury seen in Chance fractures, neurologic deficits are less common than with other serious thoracolumbar spine injuries such as burst fractures or fracture-dislocations of the spine.
Radiologic
 Though unstable injuries, Chance fractures usually occur in the absence of neurologic compromise and can be very subtle radiographically.
Though unstable injuries, Chance fractures usually occur in the absence of neurologic compromise and can be very subtle radiographically.
 Chance fractures can be difficult to diagnose when undue emphasis is placed on the lateral view of the spine. The degree of vertebral compression may be slight, giving the false impression of a simple compression fracture. Regardless of the site of trauma, it is imperative to give equal time to the inspection of the AP view and lateral radiographs in spine trauma. This is particularly true for Chance fractures in which the posterior element component is often quite impressive on the AP view while unappreciated on the lateral view.
Chance fractures can be difficult to diagnose when undue emphasis is placed on the lateral view of the spine. The degree of vertebral compression may be slight, giving the false impression of a simple compression fracture. Regardless of the site of trauma, it is imperative to give equal time to the inspection of the AP view and lateral radiographs in spine trauma. This is particularly true for Chance fractures in which the posterior element component is often quite impressive on the AP view while unappreciated on the lateral view.
 Vertical separation of the spinous processes in the setting of a Chance fracture can often be detected on the AP radiograph, as the involved vertebral body may appear relatively lucent when the spinous processes are displaced such that they are no longer superimposed on the vertebral body (the “empty vertebral body sign”).
Vertical separation of the spinous processes in the setting of a Chance fracture can often be detected on the AP radiograph, as the involved vertebral body may appear relatively lucent when the spinous processes are displaced such that they are no longer superimposed on the vertebral body (the “empty vertebral body sign”).
 Simple compression fractures of the spine are common, and although they produce severe back pain, they do not cause neurologic injury. Simple compression fractures with <25% loss in vertical body height can be treated conservatively; additional imaging is not needed.
Simple compression fractures of the spine are common, and although they produce severe back pain, they do not cause neurologic injury. Simple compression fractures with <25% loss in vertical body height can be treated conservatively; additional imaging is not needed.
 Compression fractures >25% can be deceptive on radiographs, and CT can be helpful in excluding retropulsion of fragments, a finding that is commonly underestimated from radiograph analysis.
Compression fractures >25% can be deceptive on radiographs, and CT can be helpful in excluding retropulsion of fragments, a finding that is commonly underestimated from radiograph analysis.
 The fracture line in a Chance fracture often lies within the plane of an axial CT; therefore, sagittal and/or coronal reformatted images often provide better visualization of the fracture morphology.
The fracture line in a Chance fracture often lies within the plane of an axial CT; therefore, sagittal and/or coronal reformatted images often provide better visualization of the fracture morphology.
 Burst fractures, regardless of their location in the spine, imply an axial load with radial dispersion of fracture fragments. An increase in the interpediculate distance on the frontal radiograph is a key finding. This further emphasizes the importance of inspecting the posterior elements on the anteroposterior radiograph in spinal trauma. CT and sometimes MRI are used in the evaluation of burst injuries due to the high propensity for spinal canal compromise.
Burst fractures, regardless of their location in the spine, imply an axial load with radial dispersion of fracture fragments. An increase in the interpediculate distance on the frontal radiograph is a key finding. This further emphasizes the importance of inspecting the posterior elements on the anteroposterior radiograph in spinal trauma. CT and sometimes MRI are used in the evaluation of burst injuries due to the high propensity for spinal canal compromise.
 It is not uncommon for Chance fractures to be accompanied by a burst fracture component; this finding can have significance in the management of these fractures due to the presence of osseous retropulsion related to the burst component.
It is not uncommon for Chance fractures to be accompanied by a burst fracture component; this finding can have significance in the management of these fractures due to the presence of osseous retropulsion related to the burst component.
SUGGESTED READING
Bernstein MP, Mirvis SE, Shanmuganathan K. Chance-type fractures of the thoracolumbar spine: Imaging analysis in 53 patients. Am J Roentgenol 2006;187:859–868.
Berquist TH. Imaging of Orthopedic Trauma. New York, NY: Raven, 1992:169–194.
Harris JH, Harris WH, Novelline RA. The Radiology of Emergency Medicine. Baltimore, MD: Williams & Wilkins, 1993:247–280.
Rogers LF, Daffner RH. The thoracic and lumbar spine. Radiology of Skeletal Trauma (3rd ed). New York: Churchill Livingstone, 2002:521–525.
EMILY N.
VINSON
HISTORY
A 13-year-old boy with a 1-month history of progressive left shoulder pain and swelling.
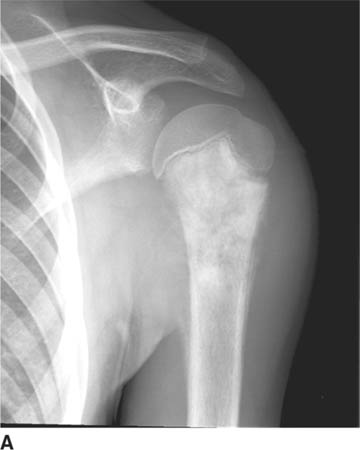
 FIGURE 5-7A Anteroposterior radiograph of the left shoulder. There is an aggressive-appearing, predominantly sclerotic lesion in the proximal left humeral metaphysis, with cloud-like areas of increased density in the interosseous portion of the lesion, as well as vague increased density in the extraosseous soft tissues just medial to the lesion. There is associated aggressive-appearing periostitis along the lateral cortical margin and a Codman’s triangle at the inferomedial aspect of the lesion. The cortex is indistinct at the superomedial aspect of the lesion.
FIGURE 5-7A Anteroposterior radiograph of the left shoulder. There is an aggressive-appearing, predominantly sclerotic lesion in the proximal left humeral metaphysis, with cloud-like areas of increased density in the interosseous portion of the lesion, as well as vague increased density in the extraosseous soft tissues just medial to the lesion. There is associated aggressive-appearing periostitis along the lateral cortical margin and a Codman’s triangle at the inferomedial aspect of the lesion. The cortex is indistinct at the superomedial aspect of the lesion.
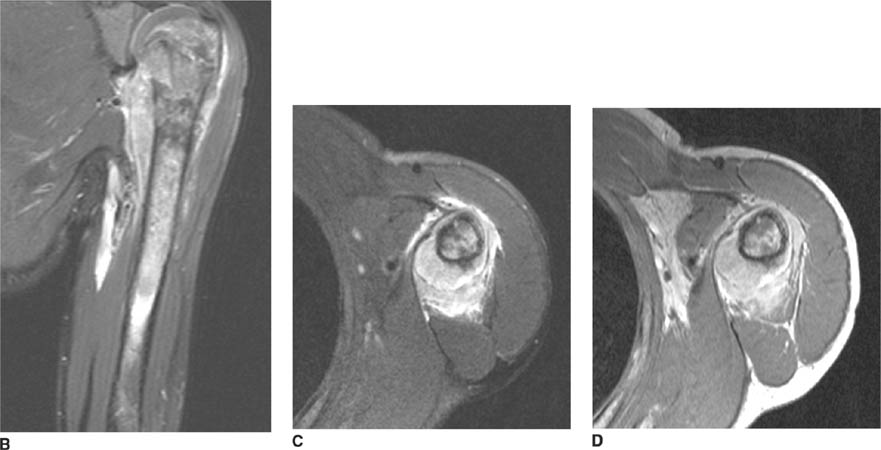
 FIGURES 5-7B, 5-7C, and 5-7D Fat-suppressed FSE T2-weighted images in the coronal (B) and axial (C) planes, and postcontrast axial Tl-weighted image (D). The lesion is of heterogeneous signal intensity on the T2-weighted images, with areas of low signal intensity corresponding to areas of mineralization. There is violation of the humeral cortex at the superomedial aspect of the mass with an associated soft tissue mass. There is edema in the soft tissues surrounding the lesion.
FIGURES 5-7B, 5-7C, and 5-7D Fat-suppressed FSE T2-weighted images in the coronal (B) and axial (C) planes, and postcontrast axial Tl-weighted image (D). The lesion is of heterogeneous signal intensity on the T2-weighted images, with areas of low signal intensity corresponding to areas of mineralization. There is violation of the humeral cortex at the superomedial aspect of the mass with an associated soft tissue mass. There is edema in the soft tissues surrounding the lesion.
DIFFERENTIAL DIAGNOSIS
 Ewing’s sarcoma: Though Ewing’s sarcoma is primarily thought of as having a permeative, lytic appearance, this tumor can elicit reactive new bone formation, resulting in a patchy sclerosis throughout the lesion, similar to what is seen in this case. This tumor is most commonly seen in the pelvis or in the metadiaphysis of a long bone, but can occur in the metaphysis of a long bone. Therefore, while not the most likely diagnosis, Ewing’s sarcoma should remain on the list of possible diagnoses in this case.
Ewing’s sarcoma: Though Ewing’s sarcoma is primarily thought of as having a permeative, lytic appearance, this tumor can elicit reactive new bone formation, resulting in a patchy sclerosis throughout the lesion, similar to what is seen in this case. This tumor is most commonly seen in the pelvis or in the metadiaphysis of a long bone, but can occur in the metaphysis of a long bone. Therefore, while not the most likely diagnosis, Ewing’s sarcoma should remain on the list of possible diagnoses in this case.
 Conventional osteosarcoma: The lesion has an aggressive appearance, with patchy areas of cloudlike increased density, aggressive periostitis, cortical destruction, and a soft tissue mass. These are features of conventional osteosarcoma. The location of the lesion in the metaphysis of a long bone and the patient’s age are also typical for osteosarcoma. Therefore, this is the most likely diagnosis.
Conventional osteosarcoma: The lesion has an aggressive appearance, with patchy areas of cloudlike increased density, aggressive periostitis, cortical destruction, and a soft tissue mass. These are features of conventional osteosarcoma. The location of the lesion in the metaphysis of a long bone and the patient’s age are also typical for osteosarcoma. Therefore, this is the most likely diagnosis.
 Osteomyelitis: Osteomyelitis can have a very aggressive appearance, and may manifest as a mixed lytic and sclerotic lesion due to bone resorption and reactive new bone formation, respectively. Aggressive periostitis can be present on radiographs, and a soft tissue mass may also be seen. However, the areas of increased density on the radiograph and the low signal intensity areas on the MR images in this case are most suggestive of oste-oid material, which would not be expected in infection. This makes osteomyelitis less likely.
Osteomyelitis: Osteomyelitis can have a very aggressive appearance, and may manifest as a mixed lytic and sclerotic lesion due to bone resorption and reactive new bone formation, respectively. Aggressive periostitis can be present on radiographs, and a soft tissue mass may also be seen. However, the areas of increased density on the radiograph and the low signal intensity areas on the MR images in this case are most suggestive of oste-oid material, which would not be expected in infection. This makes osteomyelitis less likely.
DIAGNOSIS
Conventional osteosarcoma
KEY FACTS
Clinical
 Osteosarcoma is a malignant neoplasm of bone in which the tumor cells produce osteoid matrix, and is the most common primary malignant bone tumor in children. Most patients are between the ages of 10 and 20 years at the time of diagnosis, with the vast majority under the age of 30 years. Osteosarcomas rarely occur in older patients, usually in the setting of malignant degeneration of Paget’s disease or at a site of prior external beam radiation therapy.
Osteosarcoma is a malignant neoplasm of bone in which the tumor cells produce osteoid matrix, and is the most common primary malignant bone tumor in children. Most patients are between the ages of 10 and 20 years at the time of diagnosis, with the vast majority under the age of 30 years. Osteosarcomas rarely occur in older patients, usually in the setting of malignant degeneration of Paget’s disease or at a site of prior external beam radiation therapy.
 “Conventional” osteosarcoma is the term used for the high-grade intramedullary type of osteosarcoma that occurs in the metaphysis of a long bone in children and adolescents, and is the most common type. This discussion refers to conventional osteosarcoma.
“Conventional” osteosarcoma is the term used for the high-grade intramedullary type of osteosarcoma that occurs in the metaphysis of a long bone in children and adolescents, and is the most common type. This discussion refers to conventional osteosarcoma.
 The most common sites of involvement are the distal femur, proximal tibia, and proximal humerus. Most of the tumors are intramedullary, and in a long bone the metaphysis is by far the most common location (90% to 95%). However, any portion of any bone can be involved.
The most common sites of involvement are the distal femur, proximal tibia, and proximal humerus. Most of the tumors are intramedullary, and in a long bone the metaphysis is by far the most common location (90% to 95%). However, any portion of any bone can be involved.
 Pathologic fractures are seen in 15% to 20% of cases, either at the time of presentation or during therapy.
Pathologic fractures are seen in 15% to 20% of cases, either at the time of presentation or during therapy.
 About 20% of children will have detectable metastatic disease at the time of initial diagnosis, most commonly involving the lungs as the result of hematogenous spread. Other common sites of metastatic disease include the skeleton and regional and distal lymph nodes.
About 20% of children will have detectable metastatic disease at the time of initial diagnosis, most commonly involving the lungs as the result of hematogenous spread. Other common sites of metastatic disease include the skeleton and regional and distal lymph nodes.
 Patients are usually treated with neoadjuvant courses of chemotherapy to treat potential micrometastatic disease prior to definitive resection of the tumor. Neo-adjuvent chemotherapy also often leads to maturation/ ossification of any associated soft tissue mass, allowing for an easier resection.
Patients are usually treated with neoadjuvant courses of chemotherapy to treat potential micrometastatic disease prior to definitive resection of the tumor. Neo-adjuvent chemotherapy also often leads to maturation/ ossification of any associated soft tissue mass, allowing for an easier resection.
 The advent of effective preoperative chemotherapy has markedly improved the prognosis of patients with osteosarcoma. The 5-year survival rate is 60% to 80%.
The advent of effective preoperative chemotherapy has markedly improved the prognosis of patients with osteosarcoma. The 5-year survival rate is 60% to 80%.
 Histologic response to preoperative chemotherapy is the strongest prognostic factor.
Histologic response to preoperative chemotherapy is the strongest prognostic factor.
Radiologic
 Conventional osteosarcoma usually has a very destructive and aggressive appearance on radiographs. It may be a lytic, blastic, or mixed lytic and blastic bone lesion. The initial characterization of these tumors is usually best performed with radiography.
Conventional osteosarcoma usually has a very destructive and aggressive appearance on radiographs. It may be a lytic, blastic, or mixed lytic and blastic bone lesion. The initial characterization of these tumors is usually best performed with radiography.
 The tumors are typically large, with indistinct margins, cortical destruction, aggressive periosteal reaction (such as sunburst and Codman’s triangle), and a soft tissue mass. Permeative lytic areas represent bone destruction, and cloud-like areas of increased density represent the malignant osteoid matrix produced by the tumor cells. This osteoid matrix may be visible within both the intraosseous and extraosseous portions of the tumor.
The tumors are typically large, with indistinct margins, cortical destruction, aggressive periosteal reaction (such as sunburst and Codman’s triangle), and a soft tissue mass. Permeative lytic areas represent bone destruction, and cloud-like areas of increased density represent the malignant osteoid matrix produced by the tumor cells. This osteoid matrix may be visible within both the intraosseous and extraosseous portions of the tumor.
 MR imaging does not play a major role in the initial characterization of the lesion beyond what is provided by the radiographic assessment, but plays a major role in local staging and pre-operative planning. The fact that limb-sparing resection techniques are now favored over amputation has necessitated highly accurate local staging to depict intramedullary and extramedullary disease extent prior to surgery.
MR imaging does not play a major role in the initial characterization of the lesion beyond what is provided by the radiographic assessment, but plays a major role in local staging and pre-operative planning. The fact that limb-sparing resection techniques are now favored over amputation has necessitated highly accurate local staging to depict intramedullary and extramedullary disease extent prior to surgery.
 MR imaging allows preoperative assessment of the longitudinal intramedullary extent of the neoplasm and detects epiphyseal involvement and skip metastases. The intramedullary extent of the tumor determines the resection margins. Spin echo T1-weighted sequences along the long-axis of the bone are considered the most accurate at determining the longitudinal intraosseous extent of macroscopic tumor.
MR imaging allows preoperative assessment of the longitudinal intramedullary extent of the neoplasm and detects epiphyseal involvement and skip metastases. The intramedullary extent of the tumor determines the resection margins. Spin echo T1-weighted sequences along the long-axis of the bone are considered the most accurate at determining the longitudinal intraosseous extent of macroscopic tumor.
 Skip metastases are estimated to occur in anywhere from 1% to 25% of cases of conventional osteosarcoma.
Skip metastases are estimated to occur in anywhere from 1% to 25% of cases of conventional osteosarcoma.
 By MR imaging, epiphyseal extension of osteosar-coma across an open physis is detected in up to 80% of metaphyseal osteosarcomas.
By MR imaging, epiphyseal extension of osteosar-coma across an open physis is detected in up to 80% of metaphyseal osteosarcomas.
 MR imaging also depicts the extramedullary extent of the tumor, including the size and location of any soft tissue mass and its relationship to the neurovascular bundle and compartments of the extremity, and the presence or absence of joint involvement. These features help determine whether or not limb-salvage resection will be feasible and what type of surgery will be performed.
MR imaging also depicts the extramedullary extent of the tumor, including the size and location of any soft tissue mass and its relationship to the neurovascular bundle and compartments of the extremity, and the presence or absence of joint involvement. These features help determine whether or not limb-salvage resection will be feasible and what type of surgery will be performed.
 To stage distant disease, patients also undergo radio-nuclide bone scintigraphy to evaluate for distant osseous metastatic disease and CT of the chest to evaluate for distant soft tissue metastatic disease, which is most common in the lungs.
To stage distant disease, patients also undergo radio-nuclide bone scintigraphy to evaluate for distant osseous metastatic disease and CT of the chest to evaluate for distant soft tissue metastatic disease, which is most common in the lungs.
SUGGESTED READING
Wootton-Gorges SL. MR imaging of primary bone tumors and tumor-like conditions in children. Radiol Clin N Am 2009;47:957–975.
Unni KK. Osteosarcoma of bone. J Orthop Sci 1998;3:287–294.
Saifuddin A. The accuracy of imaging in the local staging of appendicular osteosarcoma. Skeletal Radiol 2002;31:191–201.
Brisse H, Ollivier L, Edeline V, et al. Imaging of malignant tumours of the long bones in children: monitoring response to neoadjuvant chemotherapy and preoperative assessment. Pediatr Radiol 2004;34:595–605.
Suresh S, Saifuddin A. Radiological appearances of appendicular osteosar-coma: a comprehensive pictorial review. Clin Radiol 2007;62:314–323.
Murphey MD, Robbin MR, McRae GA, et al. The many faces of osteosarcoma. Radiographics 1997;17:1205–1231.
SALUTARIO J.
MARTINEZ
AND
R. LEE
COTHRAN, Jr.
HISTORY
A 16-year-old boy with persistent left ankle pain following hardware removal.
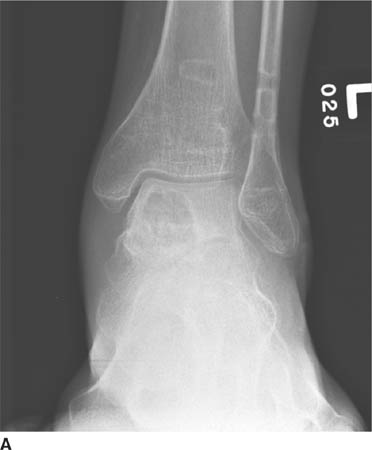
 FIGURE 5-8A Anteroposterior radiograph of the left ankle. There is a lucent expansile lesion of the medial talus, with a thin sclerotic margin. Evidence of prior hardware in the distal tibial and fibula is incidentally noted.
FIGURE 5-8A Anteroposterior radiograph of the left ankle. There is a lucent expansile lesion of the medial talus, with a thin sclerotic margin. Evidence of prior hardware in the distal tibial and fibula is incidentally noted.
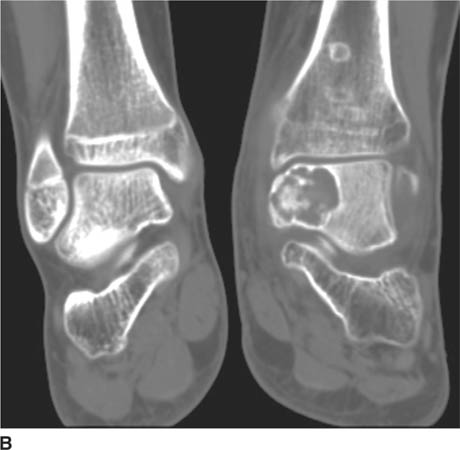
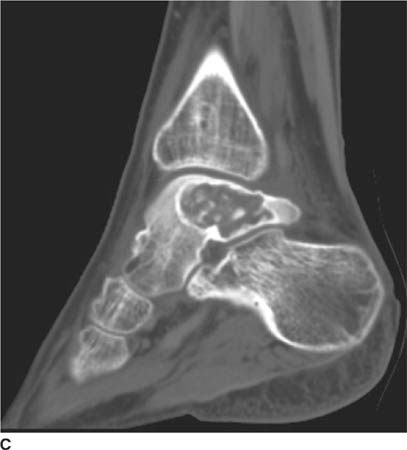
 FIGURES 5-8B and 5-8C Coronal and sagittal reformatted images from a CT scan of the left ankle. There is a well-circumscribed, mildly expansile lesion of the medial left talus with a thin sclerotic margin and containing calcified chondroid matrix. There is mild sclerosis in the adjacent marrow.
FIGURES 5-8B and 5-8C Coronal and sagittal reformatted images from a CT scan of the left ankle. There is a well-circumscribed, mildly expansile lesion of the medial left talus with a thin sclerotic margin and containing calcified chondroid matrix. There is mild sclerosis in the adjacent marrow.
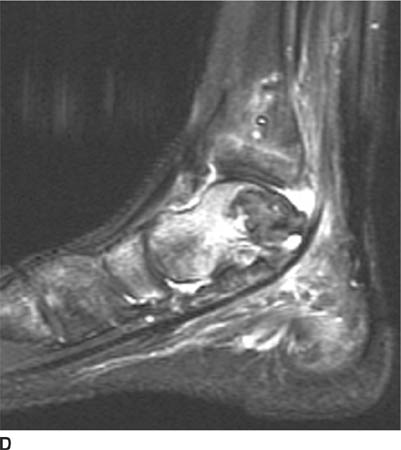
 FIGURE 5-8D Sagittal fat-suppressed FSE T2-weighted MRI of the left ankle. There is a well-circumscribed lesion of the medial talus of predominantly low signal intensity containing a few foci of increased signal intensity. The adjacent marrow and soft tissues demonstrate diffuse increased signal intensity related to edema.
FIGURE 5-8D Sagittal fat-suppressed FSE T2-weighted MRI of the left ankle. There is a well-circumscribed lesion of the medial talus of predominantly low signal intensity containing a few foci of increased signal intensity. The adjacent marrow and soft tissues demonstrate diffuse increased signal intensity related to edema.
DIFFERENTIAL DIAGNOSIS
 Brodie’s abscess: This would be a possibility based on the initial radiograph. However, the presence of a predominately low signal intensity lesion on the MRI makes this much less likely.
Brodie’s abscess: This would be a possibility based on the initial radiograph. However, the presence of a predominately low signal intensity lesion on the MRI makes this much less likely.
 Eosinophilic granuloma: Although variable in appearance, these lesions are most often metaphyseal and multifocal with less marrow edema and muscle edema than the current case demonstrates.
Eosinophilic granuloma: Although variable in appearance, these lesions are most often metaphyseal and multifocal with less marrow edema and muscle edema than the current case demonstrates.
 Aneurysmal bone cyst (ABC): The mildly expansile radiographic appearance favors this diagnosis. However, this diagnosis would not account for the solid, low signal intensity component apparent on the MR images.
Aneurysmal bone cyst (ABC): The mildly expansile radiographic appearance favors this diagnosis. However, this diagnosis would not account for the solid, low signal intensity component apparent on the MR images.
 Chondroblastoma: This tumor may involve the talus, and can have an aneurysmal component with edema of adjacent muscle and marrow. The radiographic and MRI features and the patient’s age are most consistent with a chondroblastoma.
Chondroblastoma: This tumor may involve the talus, and can have an aneurysmal component with edema of adjacent muscle and marrow. The radiographic and MRI features and the patient’s age are most consistent with a chondroblastoma.
 Giant cell tumor (GCT): The location of this mass is reasonable for a GCT. However, the presence of a sclerotic margin on the radiograph, the presence of internal matrix, and the patient’s young age make this diagnosis unlikely.
Giant cell tumor (GCT): The location of this mass is reasonable for a GCT. However, the presence of a sclerotic margin on the radiograph, the presence of internal matrix, and the patient’s young age make this diagnosis unlikely.
DIAGNOSIS
Chondroblastoma of the medial talus
KEY FACTS
Clinical
 Chondroblastoma is a benign cartilaginous tumor comprising <1% of all primary bone tumors.
Chondroblastoma is a benign cartilaginous tumor comprising <1% of all primary bone tumors.
 Its incidence is intermediate among other benign cartilaginous tumors; it is more common than chon-dromyxoid fibroma but rarer than enchondroma or osteochondroma.
Its incidence is intermediate among other benign cartilaginous tumors; it is more common than chon-dromyxoid fibroma but rarer than enchondroma or osteochondroma.
 The second decade of life is the most common age of presentation (70%). About 90% of chondroblastomas present between the ages of 5 and 25 years. The male-to-female ratio has been variably reported at ranges from 1.4:1 to 2.4:1.
The second decade of life is the most common age of presentation (70%). About 90% of chondroblastomas present between the ages of 5 and 25 years. The male-to-female ratio has been variably reported at ranges from 1.4:1 to 2.4:1.
 Symptoms are nonspecific and include pain referred to the joint adjacent to the lesion.
Symptoms are nonspecific and include pain referred to the joint adjacent to the lesion.
 Curettage and bone grafting is the treatment of choice. However, case reports have been published suggesting radiofrequency ablation of chondroblastomas as an alternative, less invasive treatment in select cases.
Curettage and bone grafting is the treatment of choice. However, case reports have been published suggesting radiofrequency ablation of chondroblastomas as an alternative, less invasive treatment in select cases.
 Local recurrence can occur following surgery. However, malignant transformation is rare.
Local recurrence can occur following surgery. However, malignant transformation is rare.
Radiologic
 Chondroblastoma is a geographic lucent lesion with thin sclerotic margins arising eccentrically in an epiph-ysis or apophysis, with or without extension into the metaphysis.
Chondroblastoma is a geographic lucent lesion with thin sclerotic margins arising eccentrically in an epiph-ysis or apophysis, with or without extension into the metaphysis.
 The most common sites of involvement include the epiphyses of the distal femur, proximal tibia, proximal humerus, and apophysis of the greater trochanter.
The most common sites of involvement include the epiphyses of the distal femur, proximal tibia, proximal humerus, and apophysis of the greater trochanter.
 Detectable punctate calcified chondroid matrix is found in about 50% of patients.
Detectable punctate calcified chondroid matrix is found in about 50% of patients.
 Up to 60% of patients have benign-appearing perios-teal reaction away from the lesion, often in the adjacent metadiaphysis in the case of epiphyseal lesions.
Up to 60% of patients have benign-appearing perios-teal reaction away from the lesion, often in the adjacent metadiaphysis in the case of epiphyseal lesions.
 On MRI, chondroblastomas have a lobulated margin. The signal intensity characteristics are distinct from those of hyaline cartilage (i.e., enchondroma). Chondro-blastomas appear homogeneously isointense to muscle on T1-weighted images and have variable signal intensity on T2-weighted images, often with some intermediate to low signal intensity. There frequently is increased T2 signal within the surrounding marrow and soft tissues. In the case of an epiphyseal lesion, there may also be a joint effusion in the adjacent joint. In 10% to 20% of chondroblastomas there may be an associated “secondary” ABC characterized by scattered foci of high signal intensity on T2-weighted images, potentially with fluid levels, along with expansion of the bone.
On MRI, chondroblastomas have a lobulated margin. The signal intensity characteristics are distinct from those of hyaline cartilage (i.e., enchondroma). Chondro-blastomas appear homogeneously isointense to muscle on T1-weighted images and have variable signal intensity on T2-weighted images, often with some intermediate to low signal intensity. There frequently is increased T2 signal within the surrounding marrow and soft tissues. In the case of an epiphyseal lesion, there may also be a joint effusion in the adjacent joint. In 10% to 20% of chondroblastomas there may be an associated “secondary” ABC characterized by scattered foci of high signal intensity on T2-weighted images, potentially with fluid levels, along with expansion of the bone.
SUGGESTED READING
Dahlin DC, Ivins JC. Benign chondroblastoma: a study of 125 cases. Cancer 1972;30:401–413.
Erickson JK, Rosenthal DI, Zaleske DJ, et al. Primary treatment of chondroblastoma with percutaneous radiofrequency heat ablation: report of three cases. Radiology 2001;221:463–468.
Petsas T, Megas P, Papathanassiou Z. Radiofrequency ablation of two femoral head chondroblastomas. Eur J Radiol 2007;63:63–67.
Resnick D. Diagnosis of Bone and Joint Disorders (4th ed). Philadelphia, PA: Saunders, 2002:3850–3866.
Weatherall, PT, Maale GE, Mendelsohn DB, et al. Chondroblastoma: classic and confusing appearance at MR imaging. Radiology 1994;190: 467–474.
CHARLES E.
SPRITZER
HISTORY
A 28-year-old man with an acute injury to the knee incurred when standing from a crouched position.
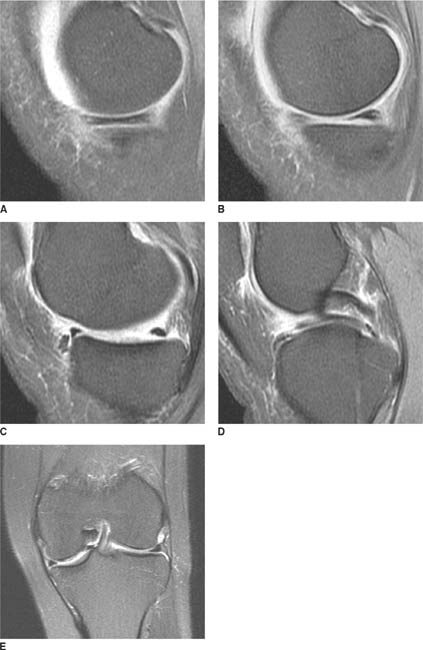
 FIGURES 5-9A-5-9E Sagittal fat suppressed proton density MR images through the medial compartment of the knee (A through D). There is deficiency of the body of the medial meniscus, evidenced by the lack of two full body segments (A and B), known as the “absent bow tie sign.” A “double-anterior horn sign” is seen (C), with the true anterior horn located just anterior to a portion of the displaced meniscal tissue. A “double-PCL sign” is seen in the intercondylar notch, due to displaced meniscal tissue lying just beneath and anterior to the PCL (D). Coronal fat suppressed FSE T2-weighted MR image of the knee (E). The body of the medial meniscus appears truncated. There is also a low signal intensity meniscal fragment seen displaced from the body of the meniscus within the intercondylar notch, lying next to the ACL and just beneath the PCL (E).
FIGURES 5-9A-5-9E Sagittal fat suppressed proton density MR images through the medial compartment of the knee (A through D). There is deficiency of the body of the medial meniscus, evidenced by the lack of two full body segments (A and B), known as the “absent bow tie sign.” A “double-anterior horn sign” is seen (C), with the true anterior horn located just anterior to a portion of the displaced meniscal tissue. A “double-PCL sign” is seen in the intercondylar notch, due to displaced meniscal tissue lying just beneath and anterior to the PCL (D). Coronal fat suppressed FSE T2-weighted MR image of the knee (E). The body of the medial meniscus appears truncated. There is also a low signal intensity meniscal fragment seen displaced from the body of the meniscus within the intercondylar notch, lying next to the ACL and just beneath the PCL (E).
DIFFERENTIAL DIAGNOSIS
 Prior arthroscopic meniscectomy: While deficiency of the meniscus is often seen following partial menis-cectomy, meniscal material in the intercondylar notch following an arthroscopy would not be expected. Furthermore, the patient has no history of arthroscopic surgery.
Prior arthroscopic meniscectomy: While deficiency of the meniscus is often seen following partial menis-cectomy, meniscal material in the intercondylar notch following an arthroscopy would not be expected. Furthermore, the patient has no history of arthroscopic surgery.
 Bucket-handle meniscal tear with displaced fragment: The body of the medial meniscus is truncated and is in direct continuity with displaced meniscal material in the intercondylar notch. This is the best diagnosis.
Bucket-handle meniscal tear with displaced fragment: The body of the medial meniscus is truncated and is in direct continuity with displaced meniscal material in the intercondylar notch. This is the best diagnosis.
 Loose osteochondral fragment: The linear appearance of the loose fragment is more in keeping with a menis-cal fragment than osteochondral bodies, which are typically round or oval in shape. In addition, no osteo-chondral donor site is seen on the available images.
Loose osteochondral fragment: The linear appearance of the loose fragment is more in keeping with a menis-cal fragment than osteochondral bodies, which are typically round or oval in shape. In addition, no osteo-chondral donor site is seen on the available images.
DIAGNOSIS
Bucket-handle tear of the medial meniscus, with a displaced fragment of meniscal tissue
KEY FACTS
Clinical
 A bucket-handle tear consists of a vertical or longitudinal tear extending around a portion of the circumference of the meniscus, usually starting in the posterior horn and extending longitudinally through the body, with variable extension into the anterior horn. The tear is typically located in the periphery of the meniscus and creates an inner fragment of meniscal tissue (the “handle”) that has a tendency to displace away from the remainder of the meniscus (the “bucket”), often displacing into the intercondylar notch.
A bucket-handle tear consists of a vertical or longitudinal tear extending around a portion of the circumference of the meniscus, usually starting in the posterior horn and extending longitudinally through the body, with variable extension into the anterior horn. The tear is typically located in the periphery of the meniscus and creates an inner fragment of meniscal tissue (the “handle”) that has a tendency to displace away from the remainder of the meniscus (the “bucket”), often displacing into the intercondylar notch.
 This type of tear can occur in either the medial or lateral meniscus. Occasionally, concurrent bucket-handle tears of both the medial and lateral menisci may exist.
This type of tear can occur in either the medial or lateral meniscus. Occasionally, concurrent bucket-handle tears of both the medial and lateral menisci may exist.
 This type of meniscal injury may be associated with rotation of either the femur or the tibia. Clinical history is usually a simple twisting injury or an injury sustained during crouching. Some patients have no known injury.
This type of meniscal injury may be associated with rotation of either the femur or the tibia. Clinical history is usually a simple twisting injury or an injury sustained during crouching. Some patients have no known injury.
 Occasionally, bucket-handle meniscal tears are seen in association with anterior cruciate ligament (ACL) tears.
Occasionally, bucket-handle meniscal tears are seen in association with anterior cruciate ligament (ACL) tears.
 Patients often present clinically with a locked knee or with an inability to fully extend the knee. Others present with a history of a locked knee at the time of the initial injury that has subsequently become unlocked. Some patients have no history of locking.
Patients often present clinically with a locked knee or with an inability to fully extend the knee. Others present with a history of a locked knee at the time of the initial injury that has subsequently become unlocked. Some patients have no history of locking.
Radiologic
 A thinned and somewhat truncated appearance to the body of the meniscus extending into the posterior horn is characteristic. There may be foreshortening of the posterior horn without a history of prior arthroscopy. Visualization of a displaced fragment of meniscal tissue is key in making the diagnosis of a bucket-handle tear on MR imaging. Careful examination of the intercondy-lar notch typically reveals the torn and displaced inner component of the meniscus. This is often seen beneath the ACL or posterior cruciate ligament (PCL) (as in this case, Figures 5-12D and 5-12E).
A thinned and somewhat truncated appearance to the body of the meniscus extending into the posterior horn is characteristic. There may be foreshortening of the posterior horn without a history of prior arthroscopy. Visualization of a displaced fragment of meniscal tissue is key in making the diagnosis of a bucket-handle tear on MR imaging. Careful examination of the intercondy-lar notch typically reveals the torn and displaced inner component of the meniscus. This is often seen beneath the ACL or posterior cruciate ligament (PCL) (as in this case, Figures 5-12D and 5-12E).
 Other imaging findings described in patients with bucket-handle tears of the meniscus include the absent bow tie sign, the double-anterior horn sign, and the double-PCL sign.
Other imaging findings described in patients with bucket-handle tears of the meniscus include the absent bow tie sign, the double-anterior horn sign, and the double-PCL sign.
 The absent bow tie sign occurs because of the deficiency of the meniscal body caused by displacement of the torn inner margin of the meniscus. Normally on sagittal images, assuming a slice thickness of 4 to 5 mm, the meniscal body will be visible on two consecutive images, and resembles a bow tie. Seeing no body segment or only one body segment suggests either prior partial meniscectomy, a small meniscus, a radial tear of the meniscal body, severe degenerative arthritis, or a bucket-handle tear (Figures 5-12A and 5-12B). Therefore, the presence of this sign prompts a careful search for other evidence of a bucket-handle tear, such as a displaced fragment of meniscal tissue.
The absent bow tie sign occurs because of the deficiency of the meniscal body caused by displacement of the torn inner margin of the meniscus. Normally on sagittal images, assuming a slice thickness of 4 to 5 mm, the meniscal body will be visible on two consecutive images, and resembles a bow tie. Seeing no body segment or only one body segment suggests either prior partial meniscectomy, a small meniscus, a radial tear of the meniscal body, severe degenerative arthritis, or a bucket-handle tear (Figures 5-12A and 5-12B). Therefore, the presence of this sign prompts a careful search for other evidence of a bucket-handle tear, such as a displaced fragment of meniscal tissue.
 The double-anterior horn sign describes the fact that the displaced meniscal tissue is often located next to the normal anterior horn in the horizontal plane. On sagittal images, there is the appearance of two anterior horns, one immediately anterior to the other. In this situation, the more anterior of the two apparent anterior horns is the true anterior horn, and the more posterior is the displaced meniscal tissue (Figure 5-12C).
The double-anterior horn sign describes the fact that the displaced meniscal tissue is often located next to the normal anterior horn in the horizontal plane. On sagittal images, there is the appearance of two anterior horns, one immediately anterior to the other. In this situation, the more anterior of the two apparent anterior horns is the true anterior horn, and the more posterior is the displaced meniscal tissue (Figure 5-12C).
 The double-PCL sign describes a displaced maniscal fragment located anterior and parallel to the under-surface of the PCL on sagittal images (Figure 5-12D). In the setting of an intact ACL, only a medial meniscal bucket-handle tear can produce a double-PCL sign. In the setting of an acute or chronic ACL tear, either a medial or lateral meniscus bucket-handle tear can produce this sign.
The double-PCL sign describes a displaced maniscal fragment located anterior and parallel to the under-surface of the PCL on sagittal images (Figure 5-12D). In the setting of an intact ACL, only a medial meniscal bucket-handle tear can produce a double-PCL sign. In the setting of an acute or chronic ACL tear, either a medial or lateral meniscus bucket-handle tear can produce this sign.
 Tears may also be present within the displaced handle.
Tears may also be present within the displaced handle.
SUGGESTED READING
Aydingoz U, Firat AK, Atay OA, Doral MN. MR imaging of meniscal bucket-handle tears: a review of signs and their relation to arthroscopic classification. Eur Radiol 2003;13:618–625.
Dorsay TA, Helms CA. Bucket-handle meniscal tears of the knee: sensitivity and specificity of MRI signs. Skeletal Radiol 2003;32:266–272.
Helms CA, Laorr A, Cannon WD Jr. The absent bow tie sign in bucket-handle tears of the menisci in the knee. Am J Roentgenol 1998;170:57–61.
Shakespeare DT, Rigby HS. The bucket-handle tear of the meniscus: a clinical and arthrographic study. J Bone Joint Surg (Br) 1983;65-B:383–387.
CHARLES E.
SPRITZER
HISTORY
A 65-year-old man with 6 months of shoulder pain.
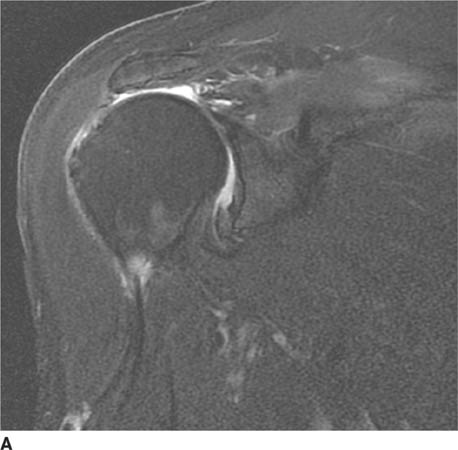
 FIGURE 5-10A Oblique coronal fat-suppressed FSE T2-weighted MR image of the shoulder. There is fluid signal intensity in the expected region of the supraspinatus tendon, with abrupt termination of the tendon several centimeters medial to its expected insertion site on the humeral head. There is mild T2-signal abnormality noted in the supraspinatus muscle belly, consistent with edema.
FIGURE 5-10A Oblique coronal fat-suppressed FSE T2-weighted MR image of the shoulder. There is fluid signal intensity in the expected region of the supraspinatus tendon, with abrupt termination of the tendon several centimeters medial to its expected insertion site on the humeral head. There is mild T2-signal abnormality noted in the supraspinatus muscle belly, consistent with edema.
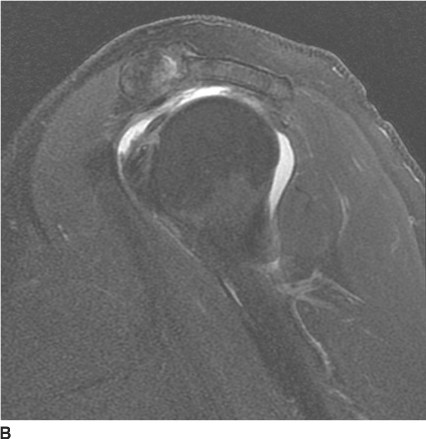
 FIGURE 5-10B Oblique sagittal fat-suppressed FSE T2-weighted MR image demonstrates nonvisualization of the supraspinatus and infraspinatus tendons and fluid extending from the joint space into the subacromial-subdeltoid bursa.
FIGURE 5-10B Oblique sagittal fat-suppressed FSE T2-weighted MR image demonstrates nonvisualization of the supraspinatus and infraspinatus tendons and fluid extending from the joint space into the subacromial-subdeltoid bursa.
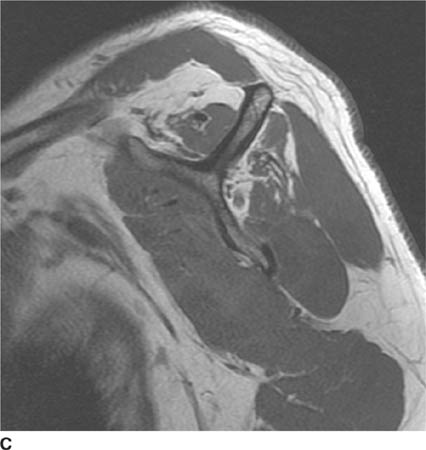
 FIGURE 5-10C Oblique sagittal Tl-weighted MR image of the shoulder, at the level of the rotator cuff muscle bellies. The supraspinatus and infraspinatus muscle bellies are small; while some of this is likely due to retraction, there is also likely a component of size atrophy. In addition, there is mild fatty infiltration of the infraspinatus muscle belly.
FIGURE 5-10C Oblique sagittal Tl-weighted MR image of the shoulder, at the level of the rotator cuff muscle bellies. The supraspinatus and infraspinatus muscle bellies are small; while some of this is likely due to retraction, there is also likely a component of size atrophy. In addition, there is mild fatty infiltration of the infraspinatus muscle belly.
 Full thickness tear of the rotator cuff tendon: The findings are consistent with this diagnosis. There is abrupt termination of the low signal intensity supraspi-natus tendon at the level of the glenoid, with high signal intensity equaling that of fluid in the expected position of the tendon. This is associated with some retraction of the muscle and tendon. In addition, fluid is seen in the subacromial/subdeltoid bursa.
Full thickness tear of the rotator cuff tendon: The findings are consistent with this diagnosis. There is abrupt termination of the low signal intensity supraspi-natus tendon at the level of the glenoid, with high signal intensity equaling that of fluid in the expected position of the tendon. This is associated with some retraction of the muscle and tendon. In addition, fluid is seen in the subacromial/subdeltoid bursa.
 Partial tear of the rotator cuff tendon: In the case shown, a full thickness rather than a partial thickness tear is present, as evidenced by high signal intensity traversing the entire thickness of the supraspinatus tendon. The abrupt cut-off of the low signal intensity in the normal tendon and the presence of retraction imply a full thickness, not partial thickness, tear.
Partial tear of the rotator cuff tendon: In the case shown, a full thickness rather than a partial thickness tear is present, as evidenced by high signal intensity traversing the entire thickness of the supraspinatus tendon. The abrupt cut-off of the low signal intensity in the normal tendon and the presence of retraction imply a full thickness, not partial thickness, tear.
 Tendinosis: While tendinosis may show slightly increased signal intensity on T2-weighted images, it should not be equal to the signal intensity of fluid and usually does not involve the entire tendon width.
Tendinosis: While tendinosis may show slightly increased signal intensity on T2-weighted images, it should not be equal to the signal intensity of fluid and usually does not involve the entire tendon width.
 Bursitis: Bursitis could produce fluid in the subacro-mion-subdeltoid bursa, but the fluid would not be expected to communicate with the glenohumeral space in the presence of an intact rotator cuff tendon.
Bursitis: Bursitis could produce fluid in the subacro-mion-subdeltoid bursa, but the fluid would not be expected to communicate with the glenohumeral space in the presence of an intact rotator cuff tendon.
DIAGNOSIS
Full thickness tear of the rotator cuff tendon
KEY FACTS
Clinical
 The rotator cuff functions as a shoulder abductor and rotator, as well as a dynamic stabilizer of the glenohu-meral joint. Clinically, rotator cuff tears may be associated with loss of shoulder strength and stability.
The rotator cuff functions as a shoulder abductor and rotator, as well as a dynamic stabilizer of the glenohu-meral joint. Clinically, rotator cuff tears may be associated with loss of shoulder strength and stability.
 The pathogenesis and natural history of rotator cuff tears have not been fully elucidated. Codman believed that a combination of tendinitis and trauma occurring in a degenerated tendon leads to rotator cuff tears (“intrinsic” theory). Neer believed in the importance of impingement of the cuff beneath the coracoacro-mial arch (“extrinsic” theory), and that impingement and rotator cuff tears represent a continuum from less severe to most severe injury. Most authors now believe that the pathogenesis of cuff tears involves two main mechanisms: subacromial impingement and intrinsic degeneration of the tendon, perhaps due to repeated trauma and hypovascularity. Rotator cuff tears can also be seen following acute trauma.
The pathogenesis and natural history of rotator cuff tears have not been fully elucidated. Codman believed that a combination of tendinitis and trauma occurring in a degenerated tendon leads to rotator cuff tears (“intrinsic” theory). Neer believed in the importance of impingement of the cuff beneath the coracoacro-mial arch (“extrinsic” theory), and that impingement and rotator cuff tears represent a continuum from less severe to most severe injury. Most authors now believe that the pathogenesis of cuff tears involves two main mechanisms: subacromial impingement and intrinsic degeneration of the tendon, perhaps due to repeated trauma and hypovascularity. Rotator cuff tears can also be seen following acute trauma.
 Asymptomatic rotator cuff tears are not uncommon, especially in elderly individuals.
Asymptomatic rotator cuff tears are not uncommon, especially in elderly individuals.
 Rotator cuff tears may be treated conservatively or surgically. Indications for surgical management of rotator cuff disease are unclear, in part because the natural history of this disease process is poorly understood. Treatment decisions are based on a number of factors, including the patient’s symptoms and the chronicity of the injury.
Rotator cuff tears may be treated conservatively or surgically. Indications for surgical management of rotator cuff disease are unclear, in part because the natural history of this disease process is poorly understood. Treatment decisions are based on a number of factors, including the patient’s symptoms and the chronicity of the injury.
Radiologic
 MRI, MR arthrography, and ultrasound are very sensitive and specific in the diagnosis of full thickness rotator cuff tears. MR arthrography detects partial thickness rotator cuff tears with greater accuracy than either MRI or ultrasound. Both MRI and MR arthrography have the advantage of allowing the evaluation of other structures in the shoulder, such as the labrum and cartilage, which are potential contributors to shoulder pain.
MRI, MR arthrography, and ultrasound are very sensitive and specific in the diagnosis of full thickness rotator cuff tears. MR arthrography detects partial thickness rotator cuff tears with greater accuracy than either MRI or ultrasound. Both MRI and MR arthrography have the advantage of allowing the evaluation of other structures in the shoulder, such as the labrum and cartilage, which are potential contributors to shoulder pain.
 Full thickness rotator cuff tears extend from the articular surface of the cuff tendon to the bursal surface. Partial thickness tears usually involve either the articular surface or the bursal surface. Interstitial tears are partial thickness tears that occur within the substance of the cuff tendon and do not involve either surface. The majority of rotator cuff tears originate in the supraspi-natus tendon.
Full thickness rotator cuff tears extend from the articular surface of the cuff tendon to the bursal surface. Partial thickness tears usually involve either the articular surface or the bursal surface. Interstitial tears are partial thickness tears that occur within the substance of the cuff tendon and do not involve either surface. The majority of rotator cuff tears originate in the supraspi-natus tendon.
 On MRI, a full thickness tear is diagnosed when there is discontinuity of the cuff fibers and fluid signal extends from the articular surface of the cuff into the subacro-mial/subdeltoid bursa. Secondary signs include tendon retraction, fluid in the subacromial/subdeltoid bursa, and muscle atrophy. Full thickness tears are usually classified according to size.
On MRI, a full thickness tear is diagnosed when there is discontinuity of the cuff fibers and fluid signal extends from the articular surface of the cuff into the subacro-mial/subdeltoid bursa. Secondary signs include tendon retraction, fluid in the subacromial/subdeltoid bursa, and muscle atrophy. Full thickness tears are usually classified according to size.
 MR imaging of the shoulder in the setting of rotator cuff tear can provide useful information to the orthopedic surgeon including tear dimensions, degree of tendon retraction, presence of muscle atrophy and/or fatty infiltration, and depth of partial thickness tears.
MR imaging of the shoulder in the setting of rotator cuff tear can provide useful information to the orthopedic surgeon including tear dimensions, degree of tendon retraction, presence of muscle atrophy and/or fatty infiltration, and depth of partial thickness tears.
SUGGESTED READING
De Jesus JO, Parker L, Frangos AJ, Nazarian LN. Accuracy of MRI, MR arthrography, and ultrasound in the diagnosis of rotator cuff tears: a meta-analysis. Am J Roentgenol 2009;192:1701–1707.
Dunn WR, Schackman BR, Walsh C, et al. Variation in orthopaedic surgeons’ perceptions about the indications for rotator cuff surgery. J Bone Joint Surg Am 2005;87:1978–1984.
Ko JY, Huang CC, Chen WJ, et al. Pathogenesis of partial tear of the rotator cuff: a clinical and pathologic study. J Shoulder Elbow Surg 2006;15:271–278.
Morag Y, Jacobson JA, Miller B, et al. MR imaging of rotator cuff injury: what the clinician needs to know. Radiographics 2006;26:1045–1065.
Opsha O, Malik A, Baltazar R, et al. MRI of the rotator cuff and internal derangement. Eur J Radiol 2008;68:36–56.
CHARLES E.
SPRITZER
HISTORY
A 55-year-old woman with a history of bilateral hip pain.
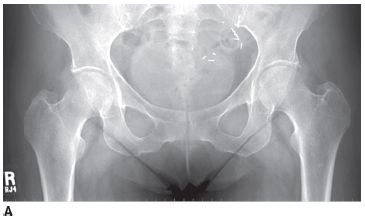
 FIGURE 5-11A Anteroposterior radiograph of the bilateral hips. There are no abnormalities noted.
FIGURE 5-11A Anteroposterior radiograph of the bilateral hips. There are no abnormalities noted.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


