Fig. 1
Representative molecular imaging methods. Reprinted from [31] with permission from Springer Science
Efforts to improve the ionization process in conventional MALDI-IMS are ongoing. Strategies to improve this process involve the following: (1) expand detectable molecular species, which is constrained by the physicochemical character of the organic matrix; (2) reduce the effect of low-molecular-weight fragments derived from the matrix material in the case of organic matrixes, which interfere with the spectra of low-molecular-weight (less than approximately 500 Da) analyte molecules in MALDI; and (3) improve spatial resolution in MALDI, which is restricted by the size of the crystals in the organic matrix [6].
A number of techniques have been investigated to address these issues [7–9]. For example, matrix-free methods, such as desorption/ionization on porous silicon (DIOS), which employs an etched silicon wafer, are capable of analyzing low-molecular-weight molecules [10]. Porous, monolithic materials have also been found to efficiently ionize small molecules [11]. Such methods typically utilize the materials’ ability to absorb laser energy and transfer it to the analyte material [12]. Using an organic matrix, a sol–gel polymeric structure into which dihydroxybenzoic acid (DHB) was covalently incorporated was reported to be used in MS analysis without the background of matrix interference spectra [13].
In this study, we developed nanoparticle laser desorption/ionization imaging mass spectrometry (nano-PALDI-IMS), a robust technique that is inexpensive, quick, and easy to perform, and that does not require specialty substrates [14, 15]. Nano-PALDI-IMS utilizes nanoparticles (NPs) composed of metal and nonmetal materials [16–18] or metals bound to fatty acid chains [14, 15], and these NPs are sprayed on tissue sections instead of organic matrixes, as in conventional MALDI-IMS (Fig. 2). We have succeeded in visualization of a vast number of metabolite species, including lipids, by employing this methodology. In this chapter, we present practical experimental nano-PALDI-IMS procedures that use four types of NPs—Ag-, Fe-, Au-, and TiO2-derived NPs—for metabolite analysis. Protocols for the preparation of NPs and their application method for IMS analysis are presented. As analysis examples, we also present our recent results of nano-PALDI-IMS analysis of metabolites in tissue specimens.
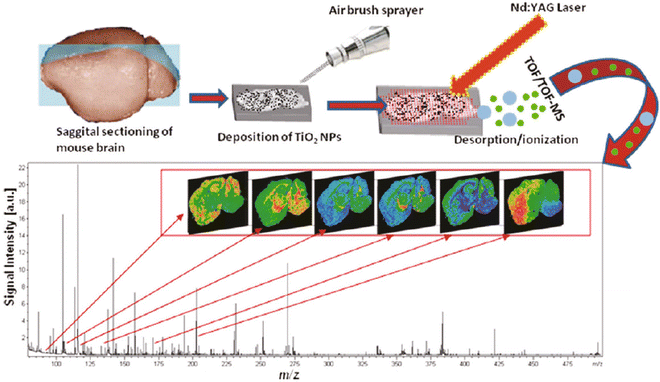

Fig. 2
Overall workflow for nano-PALDI-IMS. Tissues were sliced with a cryostat, thaw-mounted on ITO-coated glass slides, NPs were applied via an airbrush sprayer, and then, the tissue surface was analyzed by nano-PALDI-IMS. Reprinted from [16] with permission from American Chemical Society
2 Materials
2.1 Animals
1.
C57BL/6 J mice were obtained directly from a commercial breeder.
2.2 Chemicals
2.
Indium-tin-oxide (ITO)-coated glass slides.
3.
Optimal-cutting-temperature (OCT) polymer.
4.
Dry ice.
5.
Liquid nitrogen.
The following materials were used to prepare Ag NPs:
6.
n-Tetradecanoic acid sodium salt (n-C13H27COONa): 0.15 M solution in 50 % ethanol, 1 L.
7.
Stearylamine (octadecylamine).
8.
NaOH: 1 M solution in water, 150 mL.
9.
AgNO3: 1 M solution in water, 165 mL.
The following materials were used to prepare Fe NPs:
10.
FeCl2·4H2O (iron(II) chloride tetrahydride): 100 mM solution in water, 20 mL.
11.
3-Aminopropyltriethoxysilane (APTES, γ-aminopropyltriethoxysilane).
12.
Sodium acetate: 40 mM solution in methanol, 1 mL.
The following materials were used to prepare Au NPs:
13.
HAuCl4·4H2O (hydrogen tetrachloroaurate(III) tetrahydride): 1 M solution in methanol, 5 mL.
14.
Dimethyl sulfide.
15.
Oleylamine (octadecylamine).
The following materials were used to prepare TiO2 NPs:
16.
Titanium(IV) n-butoxide (titanium(IV)tetra(1-butanolate)).
17.
Nitric acid.
18.
Diammonium hydrogen citrate.
19.
Citric acid.
2.3 Instruments
20.
Cryomicrotome; Leica CM1950 cryostat (Leica Microsystems, Nussloch, Germany).
21.
Ultraflex II (Bruker Daltonics, Karlsruhe, Germany).
22.
Qstar Elite (AB SCIEX, Framingham, MA, USA).
23.
Transmission electron microscope (TEM) JEM-1010 and JEM-1230 (JEOL Ltd., Tokyo, Japan).
24.
−80 °C freezer.
25.
Magnetic stirrer with heating.
26.
Water bath.
27.
Desiccator containing a silica gel canister.
28.
Porcelain mortar.
29.
Airbrush with a 0.2 mm nozzle caliber (Procon Boy FWA Platinum; GSI Creos, Tokyo, Japan).
3 Methods
Metal NPs have enabled the MS analysis of a diverse array of molecular species [19]. Our experimental results suggest that target molecules suitable for each type of NPs in nano-PALDI-IMS are as follows: Ag NPs, Fe NPs, Au NPs, and TiO2 NPs are, respectively, suitable for the analysis of fatty acids in negative ion mode, negatively charged lipids such as sulfatides and phosphatidylserines in negative ion mode, sulfatides and gangliosides in negative ion mode, and low-molecular-weight metabolites (LMWMs) (80–500 m/z) in positive ion mode.
3.1 NP Preparation
3.1.1 Ag NPs
The Ag NPs were prepared by a coupling reaction of n-C13H27COOAg with stearylamine.
1.
The NaOH solution was added to the n-C13H27COOH solution.
2.
The AgNO3 solution was added, and the white precipitate of n-C13H27COOAg was collected and dried under reduced pressure at 60 °C.
3.
1 mmol of n-C13H27COOAg and 1 mmol of stearylamine were mixed in a one-necked flask and heated at 120 °C for 5 h.
4.
After cooling to 80 °C, methanol was added and the Ag NP precipitate was collected by filtration. The Ag NPs were washed with methanol and dried under vacuum.
5.
A 50 mg/mL solution was prepared in a microtube by adding 500 μL of hexane to the Ag NPs (see Note 1 ).
3.1.2 Fe NPs
Magnetic Fe NPs were prepared by the coupling reaction between FeCl2 with the ethoxy-group-containing APTES.
1.
The FeCl2·4H2O solution was mixed with 20 mL of APTES and stirred for 1 h at room temperature.
2.
The Fe NP precipitate was washed several times with water, dried at 80 °C, and ground in a porcelain mortar.
3.
10 mg of Fe NPs were dispersed in a sodium acetate solution in a microtube.
3.1.3 Au NPs
Au NPs were prepared by the thermolysis reaction of gold(I) sulfide with AuCl(SMe2) and the subsequent substitution of SMe2 with oleylamine.
1.
AuCl(SMe2), a white precipitate, was prepared by dissolving dimethylsulfide (10 mmol) in a HAuCl4·4H2O solution and heating [20].
2.
AuCl(SMe2) (295 mg, 1 mmol) and oleylamine (10 mmol) were mixed in a 10 mL flask by a magnetic stirrer.
3.
The transparent mixture was gradually heated to 120 °C, at which time a homogeneous purple solution formed. The solution was kept at 120 °C for 1 h, and then cooled to room temperature.
4.
5 mL of acetone and 1 mL of methanol were added and mixed, and the resulting solution was centrifuged at 400 × g for 5 min.
5.
The Au NP precipitate was collected and dried under vacuum. Then, a 1 mL Au NP solution at 50 mg/mL was prepared in hexane in a microtube.
3.1.4 TiO2 NPs
TiO2 NPs were synthesized by the acid-catalyzed hydrolysis of titanium(IV) butoxide, followed by condensation [21].
1.
17 mL of titanium(IV) n-butoxide and 8 mL of ethanol were mixed by stirring for 10 min at room temperature.
2.
375 μL of nitric acid was added dropwise to the titanium (IV) n-butoxide solution, which was cooled in an ice-containing water bath under stirring. TiO2 NPs precipitated from this solution.
3.
A 9.7 mM TiO2 NP solution was prepared by dissolving NPs into 1 mL of methanol containing 50 mM diammonium hydrogen citrate and 100 mM citric acid in a microtube (see Note 2 ).
3.1.5 Determination of Diameters of NPs
To deposit a thin layer of NPs on the target surface, NPs with sizes of less than 10 nm are required [16]. The NP mean diameters were determined by transmission electron microscopy (TEM; Fig. 3).
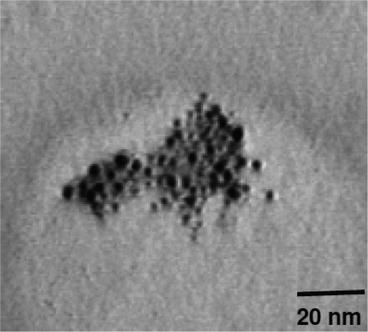

Fig. 3
Transmission electron microscopy image of TiO2 NPs. Reprinted from [16] with permission from American Chemical Society
3.2 Animal Tissue Extraction
All experiments involving mice were conducted in accordance with the protocols approved by the animal care and use committee at the research institute.
1.
Eight-week-old C57BL/6 J mice were humanely killed.
2.
Their organs were surgically collected and rapidly frozen in liquid nitrogen or powdered dry ice (see Note 3 ). The liver and retina were used for Ag NP experiments. The brain was used for Au and TiO2 NP experiments. The brains were collected within 1 min after sacrifice.
3.3 Preparation of Tissue Section
1.
Get Clinical Tree app for offline access

Tissues were affixed to an OCT polymer, taking care that no polymer was mixed into the tissue slices (Fig. 4 and see Note 4 ).
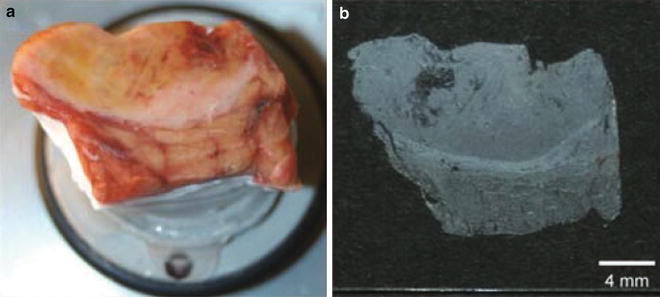

Fig. 4




Tissue sample on a cryostat sample holder. (a) The sample was affixed to the cryostat steel plate using a small amount of OCT. (b) Tissue section of 10 μm mounted onto a conductive glass slide for MALDI-IMS. Reprinted from [32] with permission from Springer Science
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree