Fig. 1
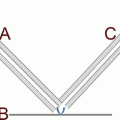
Schematic drawing of an electrochemical etching cell for porous Si preparation. Adapted with permission from ref. 7
7.
MALDI standard solution and matrix: Nominally 0.01 mM of angiotensin I, angiotensin III, and bradykinin, respectively, in water. 10 mg/mL of 2,5-dihydroxybenzoic acid (DHB) dissolved in 50:50:0.1 ACN:H2O:TFA. The peptides and matrix were mixed at a 1:1 v/v ratio.
2.2 Tissue Section Preparation
1.
Optical cutting temperature (OCT) compound.
2.
Leica Surgipath DB80 HS Premium High Profile Disposable Microtome Blade (Leica Biosystems, Buffalo Grove, IL, USA).
3.
Mouse brain tissue samples.
4.
Double-sided conductive tape.
2.3 Instrumentation
1.
EG&G Princeton Potentiostat, Model 273.
2.
Leica CM1950 cryostat (Leica Biosystems, Buffalo Grove, IL, USA).
3.
AB Sciex MALDI-TOF/TOF 5800 mass spectrometer (Framingham, MA).
4.
TOF/TOF Series Explorer Software V4.1.0.
5.
AB Sciex TOF/TOF Imaging Acquisition Software 1.0.
3 Methods
3.1 Preparation of NIMS Substrate
1.
Cut silicon wafers into 1-cm2 square-shaped chips and place in the piranha solution for 30 min to remove organic contaminants. Wash the Si chips with DI water and dry with N2.
2.
Assemble Si chip in an anodic etching cell made of Teflon, as shown in Fig. 1. A three-electrode system was used for surface etching where a Au working electrode was placed under the Si chip, and a Pt counter electrode and a Pt reference electrode were placed above the surface. Clamp the cell tight before filling with the HF etching solution (25 %). Program an EG&G Princeton Potentiostat, Model 273, to control etching current and time. The complete setup was placed in a chemical hood during substrate fabrication (see Note 2 ).
3.
Electrochemically etch the Si chip in 25 % HF solution for 30 min at a current density of 32 mA/cm2. The waste solution containing HF was carefully transferred to a pre-labeled plastic waste bottle in the hood dedicated to HF. The setup was washed with 95 % ethanol before disassembly. The produced NIMS substrate was washed again with 95 % ethanol and dried with N2 (see Note 3 ).
4.
Place the NIMS substrate in an oven at 100 °C for 5 min. Remove the substrate from oven and place it in a glass petri dish to cool down to room temperature.
5.
Place approximately 33 μL of neat BisF17 on the surface of the NIMS substrate and incubated for 30 min. Blow off excess BisF17 with N2 and then place the substrate in the oven 100 °C for 5–8 s. Repeat this blowing off of excess initiator process for a total of three times. After blowing off all excess BisF17, the substrate was washed with a generous amount of THF and dried with N2 (see Note 4 ).
6.
The substrate was stored in a sealed petri dish at room temperature until needed.
3.2 Tissue Section Preparation
1.




Snap freeze mouse brain samples in liquid N2 immediately and store at −80 °C prior to usage (see Note 5 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree