NASOPHARYNX: JUVENILE ANGIOFIBROMA
KEY POINTS
- Juvenile angiofibroma occurs at essentially one site in a particular age group, so it should always be possible to accurately make a diagnosis.
- Precise imaging is critical to a proper differential diagnosis and medical decision making.
INTRODUCTION
Etiology
Juvenile angiofibroma (JAF) is a benign vascular tumor that occurs almost exclusively in adolescent males. The etiology of JAF is unknown, but it seems to be stimulated by the hormonal surge that takes place in males at the time of adolescence. However, there are rare reports of JAF occurring in younger and older age groups. Occurrence in phenotypic females should lead to a chromosome check. JAF arises in the high posterior nasal cavity. The specific tissue of origin is not known with certainty, but it is most likely the distinctive fibrovascular stroma normally present in the nasal cavity and nasopharynx.
Prevalence and Epidemiology
JAF is a typically benign tumor presenting in the early teens but may present before age 10 years or after age 25 years. This diagnosis is in doubt after age 25 years. The tumors will stop growing and may recede once patients are in their early 20s. The tumors have androgen-specific receptors.
Clinical Presentation
The young male patient will present with unilateral nasal obstruction and/or epistaxis. This should prompt imaging by computed tomography (CT) and/or magnetic resonance (MR). Proptosis or cheek swelling are unusual presenting complaints. Eustachian tube dysfunction may result in a middle ear presentation.
Physical examination shows a polypoid mass that is indistinguishable from other polypoid nasal cavity masses, especially inflammatory fibroangiomatous polyps (Figs. 189.1 and 189.2). Proptosis and cheek swelling over the buccal space region may be present in advanced cases (Fig. 189.3). Cranial nerve palsies are very unusual, even when there is tumor at the orbital apex and along the cavernous sinus (Fig. 189.3).
PATHOPHYSIOLOGY
Anatomy
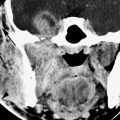
The point of origin for JAF is the mucosa of the high posterior nasal cavity near the sphenopalatine foramen (Figs. 189.4 and 189.5).
The critical anatomy is that of the posterior nasal cavity and nasopharynx. Detailed knowledge of the sphenopalatine foramen, pterygopalatine fossa, and central skull base is required for the analysis of these lesions. The relationship of the centralized areas of tumor origin to surrounding structures is pivotal. This includes the orbital apex and related orbital fissures, the pterygoid space and infratemporal fossa, and masticator and buccal spaces.
The course of basic supply vessels, including the maxillary and ascending pharyngeal arteries, must be understood. Potential supply from the vidian and cavernous segment branches of the internal carotid arteries must be understood at least in concept, if not in detail, for the diagnostic imager. A treating endovascular operator must have a thorough working knowledge of routes of supply and related potential critical anastamotic pathways.
Pathology and Patterns of Disease
Pathology
Grossly, the tumor is nonencapsulated, locally invasive with a “pushing” margin, and highly vascular. It spreads by oozing into adjacent cracks and crevices and expanding where space allows (Figs. 189.2–189.5). Regressive remodeling and erosion of bone are common features, and a permeative or moth-eaten pattern of bone destruction is highly unusual and suggests alternative etiologies.
Internally, the tumors are packed with dilated blood vessels with intervening fibrous stroma. The tumors grow nonencapsulated. The main differential diagnosis is inflammatory fibrovascular polyps (Fig. 189.1). In this age group, sarcoma and hemangioma might also be considered. CT and MR growth and morphologic patterns together with the age and gender of the patient normally make the differential diagnosis straightforward.
Patterns of Spread
Early tumors are unusual. When present, they bridge the posterior nasal cavity and nasopharynx, centered roughly at the posterior choana and pedicled to the sphenopalatine foramen (Figs. 189.4 and 189.5). At that point, the sinuses are not normally involved but may be obstructed. Growth is always along anatomic paths of least resistance; thus, continued early extension is directed toward the nasal cavity and nasopharynx and into the pterygopalatine fossa from the sphenopalatine fossa. Eventually, the tumor will fill the nasopharynx.
Continued growth from the pterygopalatine fossa expands into the infratemporal fossa, causing regressive remodeling of the posterior maxillary antral wall (Fig. 189.3). The tumor freely expands to sometimes fill the infratemporal fossa and bulges toward the cheek via the buccal space. Tumor pushes into the orbit through the inferior orbital fissure and grows intracranially through the superior and inferior orbital fissures lateral to the cavernous sinus from which it may derive blood supply. Superior extension may also occur into the basisphenoid and may involve that part of the central skull base and then sphenoid sinus (Figs. 189.2–189.5). The medial dura of the cavernous sinus may be involved from such extension. Inferior spread from the basisphenoid into the pterygoid base and between the pterygoid processes in the pterygoid fossa is common. Central skull base extension will involve the vidian canal and an appropriate supply from that artery and dural supply from the carotid siphon (Fig. 189.2H). Massive intracranial spread involving the sella is unusual.


FIGURE 189.1. Three adolescent males presenting with nosebleeds and what were thought to be juvenile angiofibromas (JAFs). A, B: Patient 1. Contrast-enhanced computed tomography (CT) study showing the lesion actually to be a vascular malformation within the soft tissues of the nasopharynx rather than JAF. In (B), the arrows show the malformation conforming to the anatomy of the fossa of Rosenmüller. C–E: Patient 2. Non–contrast-enhanced CT study showing fairly typical antrochoanal polyps with no definite evidence of penetration into the pterygopalatine fossa but a suggestion that such penetration might be present (arrow in E). There was still clinical concern that this could be a JAF, so an angiogram was done that showed this only to be a fibroangiomatous polyp. F–H: Patient 3. Magnetic resonance study showing typical antrochoanal polyps extending into the nasal cavity with no abnormality in the pterygopalatine fossa as seen on the T1-weighted images with contrast in (F) and (G) and the T2-weighted image in (H). (continued)


FIGURE 189.2. An adolescent male with nosebleeds and a polypoid mass in the posterior choana region. A: Contrast-enhanced T1-weighted (T1W) image showing an enhancing mass entering the sphenopalatine foramen to occupy the pterygopalatine fossa and spreading to the infratemporal fossa. An enlarged maxillary artery is present (white arrow), and flow voids within the lesion (arrowhead) are present. B: T2-weighted image showing the flow voids within a mass of mixed signal intensity. C: Contrast-enhanced T1W image showing the tumor entering the pterygopalatine fossa as seen in a coronal plane. D: T1W sagittal image with contrast showing the branching flow void of a vascular pattern within the polypoid nasopharyngeal mass. A particularly large vascular pedicle is shown by the arrow. E–H: An angiogram showing the typical major feeding vessel to be the maxillary artery. This is also seen in (F), where the vascular supply is seen extending across the midline and fed by nasal branches of the distal maxillary artery. In (G), there is a typical parenchymal enhancement pattern to show the angioarchitecture of these highly vascular lesions. In (H), supply from the cavernous segment of the carotid artery is present. The presence of such supply from the internal carotid artery makes complete preoperative embolization challenging and can lead to lack of control of these vessels and substantial blood loss at surgery if not appreciated.
The predominant blood supply is from the maxillary and ascending pharyngeal arteries (Figs. 189.2–189.4). Spread along the cavernous sinus dura and to the central skull base will appropriate internal carotid supply greatly complicating a surgical approach following endovascular devascularization. Once a JAF crosses the midline contralateral internal and external carotid supply is also possible (Fig. 189.2H) as a further complicating factor. The tumors do not metastasize.
IMAGING APPROACH
Techniques and Relevant Aspects
Contrast-enhanced computed tomography (CECT) and/or contrast-enhanced magnetic resonance (CEMR) begin the evaluation. CT can be done with a CT arteriogram followed by a delayed set of images. Catheter angiography often retains a central role in management and sometimes the diagnosis. MR angiography is not useful.


FIGURE 189.3. Two patients with very extensive juvenile angiofibromas (JAFs). A–E: Patient 1. A contrast-enhanced image in (A) showing the JAF filling the nasopharynx and maxillary sinus, bulging into the soft tissues of the check anteriorly. It also extends into the infratemporal fossa. In (B), the contrast-enhanced T1-weighted (T1W) image shows zones of gross necrosis within the untreated mass as well as the extent within the infratemporal fossa, breaking through the maxilla and into the soft tissues of the cheek. The T1W image in (C) shows enhancing tumor bulging into the inferior orbital fissure to spread along the posterolateral wall of the orbit and extending there from the inferior orbital fissure (arrowheads). In (D), a T2-weighted (T2W) image shows vascular flow voids (arrows). It also shows cystic changes within the mass and how well T2W magnetic resonance differentiates obstructed sinuses from those involved with the tumor. In (E), T1W images also show the flow voids due to enlarged vessels. The contrast between tumor and obstructed sinus contents is less than on the T2W image in (D). F–K: Patient 2. Contrast-enhanced T1W images showing the mass spreading to the fill the nasopharynx and posterior nasal cavity and extending into the infratemporal fossa through the sphenopalatine foramen. In (H), the solid enhancing tumor fills the nasopharynx and nasal cavity with a component displacing the posterior maxillary sinus wall as it bulges into the infratemporal fossa. In (I), a component of the tumor bulges into the sphenoid sinus, and another component (arrows) is seen extending from the inferior orbital fissure into the cavernous sinus region (arrows). Coronal images in (J) and (K) show the tumor involving the superior and inferior orbital fissure (arrows in J) with continued extension into the cavernous sinus (arrow in K).



FIGURE 189.4. Two patients to demonstrate critical growth patterns of juvenile angiofibromas (JAFs) relative to the base of the pterygoid plates and pterygopalatine fossa. A–D: Patient 1. Computed tomography (CT) and angiogram were done. In (A), the contrast-enhanced CT study shows minimal extension of tumor through the sphenopalatine fossa into the pterygopalatine fossa. In (B), bone windows show a minor amount of remodeling of the bone around the pterygopalatine fossa (arrow). More importantly, it shows direct invasion of the base of the pterygoid plates (arrowhead). Such invasion, if it goes unnoticed, can lead to persistent tumor following surgery. In (C), there is a series of four images through this critical area of potential spread showing the relationship of tumor to the pterygopalatine fossa and base of the pterygoid plates (arrowheads). In (D), the angiogram shows a typical JAF appearing as if gross total resection would be relatively simple. It had no carotid supply. However, the extension into the skull base at the base of the pterygoid plates and into the so-called pterygoid fossa makes the surgical approach challenging. E–K: Patient 2. CT study and angiogram in a patient treated surgically for a JAF with limited extension into the pterygopalatine fossa and no skull base involvement. The non–contrast-enhanced CT in (E) shows the mass in the upper nasopharynx. Bone windows in (F) show no definite remodeling in the pterygopalatine fossa; in (G), only very minimal involvement of the pterygopalatine fossa via the sphenopalatine foramen appears to be present (arrow) compared to the right side. In (H), that limited involvement in the region of the pterygopalatine fossa is confirmed, and no further skull base invasion is present (arrow). In (I) and (J), from an angiogram, the typical supply from the maxillary artery is shown. There is no carotid supply. In (K), the post embolization complete devascularization of the tumor is shown. (NOTE: This tumor was removed completely, and the patient had no recurrence.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree