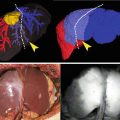
Fig. 19.1
(a) HuH-7 tumors were observed in the center of abdomen of the transplanted mice under visible light. (b) Twenty-four hour after ICG administration, HuH-7 tumors in mice exhibited uniform fluorescence under the fluorescence imaging system. When the fluorescence intensity of the background tissue was defined as 1 AU, the mean HuH-7 tumor-to-background ratio was 255 on 24 h after ICG administration
Fluorescence Histological Patterns and Attenuation Time of HuH-7 Cells
Under fluorescence microscopy, normal tissue surrounding HuH-7 tumors produced no fluorescence. All HuH-7 cells, however, were fluorescent. Fluorescence was observed in the cytoplasm of HuH-7 cells, but not in the nucleus (See Fig. 19.2a). Fluorescence of HuH-7 tumors gradually decreased over time, but retained an extremely high tumor-to-background ratio for up to 6 days. When the fluorescence intensity of the background tissue was defined as 1 AU, the mean HuH-7 tumor-to-background ratio (ICG+ NIR− and ICG+ NIR+ groups) was 255-, 100-, and 72-fold on days 0, 3, and 6, respectively (See Fig. 19.2b).
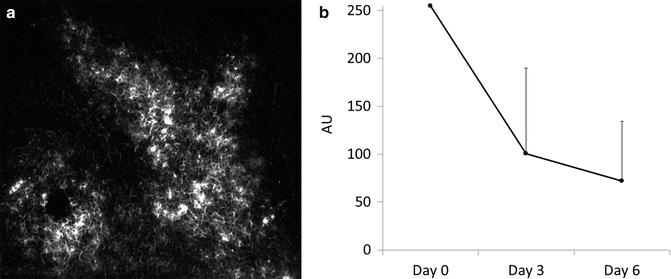

Fig. 19.2
(a) Under fluorescence microscopy, normal tissue surrounding HuH-7 tumors produced no fluorescence. All HuH-7 cells, however, were fluorescent. Fluorescence was observed in the cytoplasm of HuH-7 cells, but not in the nucleus. (b) Fluorescence in the ICG+ NIR− and ICG+ NIR+ groups gradually decreased over time, but retained an extremely high tumor-to-background ratio for up to 6 days. Under the fluorescence imaging, when the fluorescence intensity of the background tissue was defined as 1 AU, the mean HuH-7 tumor-to-background ratio was 255-, 100-, and 72-fold on days 0, 3, and 6, respectively
Pathologic Study of HuH-7 Cells Tumor 36 h After NIR Irradiation
Sections from the ICG+ NIR+ group (randomly selected and partially irradiated) were stained with hematoxylin and eosin 36 h after NIR laser exposure. At 100× magnification, a clear boundary was observed between areas with NIR laser exposure (right side) and those with no exposure (left side, See Fig. 19.3a). NIR laser exposure of HuH-7 tumors induced the following changes: pyknotic nuclei, increased intracellular spacing, and shrinkage of HuH-7 cells. These changes were more evident at 200× magnification (See Fig. 19.3b). These findings were observed not only superficially, but also in the deep parts of the tumor. HuH-7 cell behavior was not changed in the non-irradiated areas of the tumor.
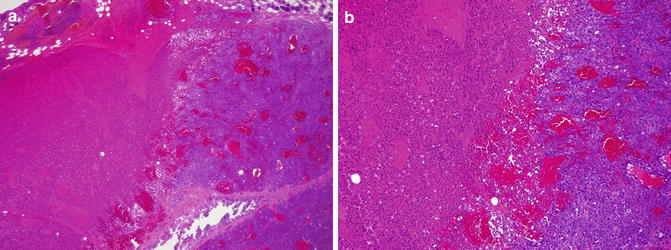

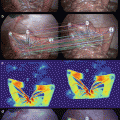
Fig. 19.3
(a) Sections from the ICG+ NIR+ group (randomly selected and partially irradiated) were stained with hematoxylin and eosin 36 h after NIR laser exposure. At 100× magnification, a clear boundary was observed between areas with NIR laser exposure (right side) and those with no exposure (left side). (b) NIR laser exposure (right side) of HuH-7 tumors induced the following changes: pyknotic nuclei, increased intracellular spacing, and shrinkage of HuH-7 cells. These changes were more evident at 200× magnification
HuH-7 Tumor Growth After NIR Laser Irradiation
Mean tumor volume in the ICG+ NIR− mice and mice receiving NIR only and no ICG (ICG− NIR+) increased steadily from 402 and 298 mm3 on day 0 to 4,272 and 3,757 mm3 by day 9, respectively (See Fig. 19.4a). In contrast, mean tumor volume in the ICG+ NIR+ group did not change between days 0 and 3 (217 and 249 mm3, respectively) and then gradually increased to 1,058 mm3 by day 9 (See Fig. 19.4b). Mean tumor volume did not differ significantly between the ICG− NIR+ and ICG+ NIR− groups (p = 0.9), but was significantly different between the ICG+ NIR+ group and both the ICG− NIR+ and ICG+ NIR− groups (p < 0.01, both; Fig. 19.4c).
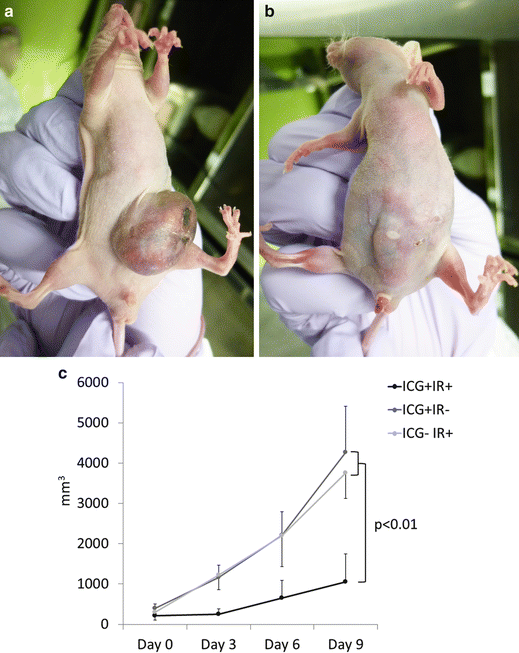

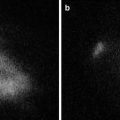
Fig. 19.4
(a) Mean tumor volume in the ICG+ NIR− and ICG− NIR+ groups increased steadily from 402 and 298 mm3 on day 0 to 4,272 and 3,757 mm3 by day 9, respectively. Large HuH-7 tumor was observed in the abdomen of the transplanted mice (ICG+ NIR− group) at day 9. (b) In contrast, mean tumor volume in the ICG+ NIR+ group did not change between days 0 and 3 (217 and 249 mm3, respectively), and gradually increased to 1,058 mm3 by day 9. Thin and obscure HuH-7 tumor was observed in the abdomen of the transplanted mice (ICG+ NIR+ group) at day 9. (c) Mean tumor volume did not differ significantly between the ICG− NIR+ and ICG+ NIR− groups (p = 0.9), but was significantly different between the ICG+ NIR+ group and both the ICG− NIR+ and ICG+ NIR− groups (p < 0.01, both)
Discussion
The findings of the present study indicated that ICG is preferentially taken up by HuH-7 human HCC cell line tumors. The tumor-to-background ratio of HuH-7 tumors, in particular, was extremely high (255:1 at 24 h after ICG administration). In our previous clinical report, we hypothesized that the human HCC was fluorescent because it retained some of the preoperatively administered ICG due to a biliary excretion disorder in the cancerous tissue [9]. Here, we present an animal model that has similar characteristics and can therefore be used to clarify the pharmacokinetics of ICG in HCC cells. The other findings of the present study indicated that PDT with NIR light irradiation suppressed HuH-7 human HCC cell line tumor growth [16].
The detailed effects of combined ICG and NIR laser light PDT is not known. At least two possible mechanisms of action, however, have been reported. ICG absorbs around 800 nm NIR light and 88 % of the absorbed light is converted to heat [10]. Second, PDT generates singlet oxygen, which causes cancer cell death [17]. Although the mechanism was not determined in the present study, we confirmed in the preliminary study that HuH-7 cell behavior was altered and tumor growth was suppressed by the combined ICG and NIR PDT. Further study needs to clarify cell death mechanism [18], using the present study model. The analysis of impaired ICG excretion and correlation biliary transporters of HuH-7 cell line also needs to elucidate strong fluorescence of this cell line tumor [19].
Regarding photosensitizer ICG, in clinical setting, combination of ICG and NIR light is used in dermatology [20] for PDT treatment of port-wine stains. However, there were no reports of ICG and NIR light use for treatment of cancer in clinical situation. According to experimental report, ICG injected directly into a HeLa cell line tumor induced strong fluorescence, but the fluorescence was limited to only a portion of the tumor because the ICG did not infiltrate the entire tumor [21]. Other studies have reported limited success using this ICG and NIR combination for the PDT treatment of several non-HCC cancer cell lines, even though none of these tumor cell lines specifically take up ICG [5, 21, 22]. Those lacks of tumor selectivity of ICG, however, could result in the PDT inducing damage to the tissue surrounding the tumor.
The present PDT for human HCC model has several advantages. First, the fluorescence has a high tumor-to-background ratio. Previously evaluated combinations of other photosensitizers, light sources, and tumor cell lines showed weak tumor selectivity, with a tumor-to-background ratio ranging from 1.4:1 to 7:1 [7, 23–26], which is substantially lower than that obtained in the present HuH-7 tumor model. The selective high tumor-to-background ratio of the present model may enable us to perform safer PDT.
Second advantage of our model is that the ICG remained in the HuH-7 tumor for a long time, maintaining a high tumor-to-background ratio for at least 6 days after ICG administration. Shafirstein and colleagues reported ICG fluorescence in a murine mammary tumor mouse model. Their model showed a lack of tumor selectivity of ICG and the fluorescence continued for only around 15 min. Therefore, NIR light irradiation had to be performed immediately after intravenous ICG administration [5]. In the present study, we examined the effect of only a single session of NIR laser irradiation. As the HuH-7 human HCC cell line remained fluorescent for a long time, several cycles of NIR laser irradiation are possible and may be more effective. On the other hand, although PDT was not performed, Ogawa and colleagues reported an interesting animal model in which florescence continued for 1–4 days in ATAC4 tumor cells with ICG conjugates of a monoclonal antibody [26].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree