Fine-needle aspiration (FNA) and core biopsy of masses in the neck predominantly include samples from thyroid nodules, parathyroids and lymph nodes. The diagnostic rate of a thyroid nodule FNA improves up to 6 passes and then does not significantly change. Thyroid FNA can be performed on patients who are anticoagulated. Appropriate transducer selection is essential for visualization of the needle. Lymph node biopsies can be additionally sampled for thyroglobulin assay to improve sensitivity for detection of recurrent carcinoma. Parathyroid FNA usually involves additional estimation of parathyroid hormone concentration in needle washouts. Biopsies of the neck are simple procedures with minimal complications.
Key points
- •
Multiple variations of fine-needle aspirations exist. Using needles between 23-gauge and 27-gauge and making 4 to 6 passes achieves the best results for most nodules.
- •
Visualization of the biopsy needle in plane with the transducer is the preferred method.
- •
Parathyroid biopsies have higher sensitivity and specificity if they include needle washout samples to estimate the parathyroid hormone (PTH) concentration.
Introduction
Ultrasonography is commonly used to guide diagnostic sampling procedures in the neck, primarily targeting thyroid nodules with sonographic findings raising suspicion for thyroid carcinoma. Lymph node diagnostic sampling using sonographic guidance has also been widely adopted and has supplanted surgical excisional biopsy in the evaluation of physically enlarged or image-detected suspicious lymph nodes in patients with known or suspected malignancy at risk for neck nodal metastases. In general, imaging-detected parathyroid adenomas can be reliably characterized based on scintigraphic and sonographic findings, so presurgical diagnostic sampling of hyperparathyroid patients with parathyroid adenomas is reserved for those infrequent instances in which the diagnosis is unclear. However, advances and improvements in ablation technique have allowed ablation treatment of parathyroid adenomas in some patients. Similarly, ablative techniques can be applied to papillary thyroid carcinoma recurrences in the thyroid surgical bed or in neck nodes. This article discusses important practical considerations in performing diagnostic sampling and ablative procedures in the neck in the context of endocrine tumors. Although most of the discussion would also be pertinent to the evaluation of other head and neck masses (including those in the parotid/salivary glands, those related to squamous cell carcinoma and other head and neck primary tumors, those related to lymphoma, and those related to soft tissue lumps and bumps), these other potential diagnostic sampling and therapeutic ablation targets are not addressed here, in keeping with the theme of endocrine imaging. After providing an overview of fine-needle sampling terminology and efficacy, including a review of the role of core biopsy for thyroid nodules, this article goes into practical details regarding our technique for performing thyroid nodule fine-needle sampling, articulates pitfalls and pearls in the application of that technique, and subsequently describes special considerations when this technique is applied to possible parathyroid adenomas or thyroid gland lymphomas. The article concludes by examining emerging ablative techniques applied to thyroid and parathyroid tumors.
Fine-needle sampling: terminology, efficacy, and quality assessment
Although the term fine-needle aspiration (FNA) has become the standard way to describe fine-needle sampling of thyroid nodules and other targets, it is important to understand that this broad term applies to 2 different variations in method, and unfortunately 1 of the variations shares the same name. When first introduced, the term aspiration biopsy reflected the method in which the operator applies suction (ie, aspiration) using a syringe, sometimes with a special syringe holder, as a fine needle is moved through a target to draw up cellular material that can then be plated out on a slide for histologic evaluation; this has been termed fine-needle aspiration sampling (FNAS) or fine-needle aspiration cytology (FNAC). A modification of this technique without using aspiration was pioneered in France in the 1980s and first described for thyroid lesions by Santos and Leiman in 1988; this has been termed fine-needle capillary sampling or fine-needle nonaspiration cytology (FNNAC). The use of the term capillary instead of aspiration is unfortunate, because it implies that capillary action is the mechanism by which the cellular material is drawn up into the needle ; in fact, the cellular material is pushed into the needle by the scraping effect of the beveled needle edge combined with the repeated excursions in the tissue, and capillary forces serve only to keep the material in the needle bore when the needle is removed. It is likely that this scraping action that loosens cellular material from the nodule architecture also plays a primary role when aspiration is applied, with the aspiration not loosening the material itself but only serving to help draw the material scraped off by the repeated needle excursions into the needle bore. In any case, the broader term fine-needle aspiration and its abbreviation FNA are commonly used to refer to either method, and this broader meaning is implied by the use of the abbreviation FNA in this article unless otherwise specified.
Comparison between the efficacy of FNAC and FNNAC has been a subject of considerable investigation. The principal problem with FNAC is that the application of aspiration increases the chance of bloody smears, which impair cytologic interpretation; this is particularly an issue with thyroid nodules, which tend to yield bloody samples with aspiration. In contrast, the principal problem with FNNAC is that operators may not scrape enough cells into the needle, either because of suboptimal technique or because of fibrotic nodules. There have been several studies that purport to compare the efficacy of FNAC and FNNAC in evaluating thyroid nodules, and a meta-analysis of those studies that eventually reviewed 1842 individual patients with 2221 samples collected by both FNAC and FNNAC showed that both techniques can be useful. The analysis concluded that the method performed likely depends on operator preference, but acknowledged that a combination approach might be best. The Consensus Statement on Thyroid FNA put forward by the Korean Society of Thyroid Radiology advocates that operators start with the FNNAC technique and continue with that technique if samples seem to be adequate, switching to the FNAC technique if initial passes yield only minimal material.
Another factor that can influence the efficacy of fine-needle sampling is needle size. Recognition that the cellular material is scraped off and not sucked out of the nodule when FNA is performed helps to understand why there is usually worse performance of FNA with larger needle size. Larger needles (larger than 23 gauge), especially when used with the aspiration method, only increase the amount of contaminating blood drawn into the needle without significantly increasing the amount of scraped cells. In contrast, as needles get thinner (smaller than 27 gauge), they may be harder to direct through overlying soft tissues and may be less effective in scraping material off the nodule, particularly if there is calcification. As a result, using a needle between 23 gauge and 27 gauge is likely to achieve the best results for most nodules.
Immediate cytopathology assessment of samples to establish sampling adequacy can positively influence FNA efficacy but has workflow drawbacks. Usually in this workflow, a cytopathologist or cytopathology technologist is requested to come with the necessary equipment (microscope) to the room in which the FNA procedure is being performed to evaluate samples as they are collected; after each sampling pass, time is taken for the cytopathologist to review the material, telling the operator to stop taking samples once sample adequacy has been confirmed. This process requires cytopathology resources that are not always available; moreover, resource coordination becomes more challenging in that the FNA procedure cannot begin until the cytopathologist is in the room, introducing workflow delays. In addition, the time taken by the cytopathologist to review the material after each pass adds time to the procedure. In contrast, a nondiagnostic result caused by specimen inadequacy adds considerably more cost because the patient may then return for another procedure or may end up going to surgical evaluation for a nodule that may prove to be benign. A study performed by Zhu and Michael revealed interesting data that highlight how procedural technique can be altered to substantially reduce nondiagnostic thyroid nodule FNA outcomes when immediate assessment of cytologic adequacy is not pursued. The nondiagnostic rate of thyroid nodule FNA for patients subdivided into groups by the number of passes taken proved to be a function of the number passes, with increasing success rates as the number of passes approached 6 ( Table 1 ). In the group of patients in whom 6 passes were made, the nondiagnostic rate was 1.4%, and this rate did not change significantly for groups with more than 6 passes.
| FNA Passes Taken | Number of Patients | Number of Nondiagnostic Results | Nondiagnostic Rate (%) |
|---|---|---|---|
| ≤3 | 20 | 5 | 25.0 |
| 4 | 100 | 11 | 11.0 |
| 5 | 115 | 6 | 5.2 |
| 6 | 67 | 1 | 1.4 |
| ≥7 | 141 | 2 | 2.1 |
This recognition that operators should optimally take 6 passes when not using immediate assessment of cytologic adequacy is important to consider when trying to understand the role of core needle biopsy (CNB) for thyroid nodules. For example, a team of Korean investigators has published several articles touting the role of CNB in the diagnosis of thyroid malignancy, concluding that core biopsy may even have a role as an initial diagnostic procedure for thyroid nodules instead of FNA. , In part, this team makes this conclusion because their analysis presumes a nondiagnostic FNA rate of 22.6%, which itself is based on practice in which operators take 3 or fewer FNA samples. With such sparse sampling practice, it is no wonder that FNA compares poorly with CNB; their 22.6% nondiagnostic rate for FNA is concordant with that predicted by the data from Zhu and Michael when taking so few samples (see Table 1 ). A more robust meta-analysis shows that there is no demonstrable role for core biopsy before FNA in the evaluation of thyroid nodules. Core biopsy might play a role in further evaluation of nodules in which initial FNA outcome is that the nodule has atypia of undetermined significance/follicular lesion of undetermined significance, but evidence suggests that using CNB instead of a second FNA for such lesions does not meaningfully change patient care, because patients typically move on to diagnostic lobectomy anyway.
Thyroid and parathyroid fine-needle aspiration: our approach
Every ultrasonography practice has site-specific variations in how patients are selected for thyroid nodule FNA and how the procedure is performed, reflecting institutional framework and resources, referring clinician desires, patient expectations, and radiologist preferences. Because it can be useful to understand how other practices perform these procedures, this article highlights our approach at Mayo Clinic Arizona.
- •
Patient selection: it is important to communicate with referring providers when selecting which nodules to sample. Although there has been wide adoption of the American College of Radiology (ACR) Thyroid Imaging Reporting and Data System (TI-RADS) algorithm to stratify the malignancy risk of thyroid nodules using sonographic features, the scheme used at Mayo Clinic Arizona (as articulated in AskMayoExpert) varies in some ways because it represents a collaboration with other stakeholders at our institution who do not agree completely with the ACR model. A full discussion of the exact algorithm in use at Mayo Clinic Arizona is beyond the scope of this article; much of the algorithm is similar to the ACR TI-RADS approach, and it uses the ACR lexicon. The important concept to convey is that ultrasonography practices should try to work with their referring providers and should communicate whatever system they are using in their reports. In our practice, we include a link to the AskMayoExpert guidelines, as well as the ACR lexicon.
- •
Scheduling notes: we have an agreement with our referring providers to schedule patients for thyroid ultrasonography and FNA when a nodule is clinically suspected. If we find a nodule that meets FNA criteria, we proceed with a biopsy without spending additional time in trying to get the order from the physician for the biopsy. This arrangement is extremely helpful to expedite evaluation of the thyroid nodule and a biopsy, if needed, efficiently.
- •
Anticoagulation status: in our estimation, thyroid FNA is safe and can be performed on patients who are anticoagulated or on aspirin. This opinion is in accordance with the Society of Interventional Radiology guidelines for quality improvement related to percutaneous needle biopsy. Anticoagulation does not significantly reduce the success rate for thyroid FNA.
- •
Patient position: in general, mild hyperextension of the neck is useful and can be achieved by using a small pillow or towel under the shoulders of the patient in a supine position. The patient’s face is turned to the opposite direction in relation to the planned biopsy site (head to left for biopsy of a nodule in right lobe and vice versa). Adequate positioning helps in stretching of the skin surface and pushes the thyroid more anteriorly, thus reducing the depth of the biopsy target ( Fig. 1 ).

Fig. 1
Positioning for biopsy. A pillow under the upper back and lower neck allows for hyperextension ( arrows ) of the neck, pushing the thyroid anteriorly and also fixing it by stretching the soft tissues.
- •
Transducer: thyroid biopsies are preferred to be performed with a high-frequency, small-footprint, hockey-stick transducer (we use a L8-18i-D transducer). This transducer lends itself well to the subtle manipulations required for adequate trajectory establishment. Sometimes a larger-footprint linear transducer may also be used. We prefer the latter when we need to use part of the transducer to exert pressure on the neck to fix a calcified or fibrous nodule that moves (sideways) when we attempt to insert the FNA needle into the nodule.
- •
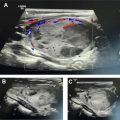
Approach: some operators always prefer a trajectory to the nodule that has a medial to lateral approach (from the tracheal side to the sternocleidomastoid muscle side), whereas others prefer a lateral to medial approach (arguing that a pathway through the hypoechoic sternocleidomastoid muscle helps visualize the needle better). Irrespective of the personal preference, the trajectory chosen should be the shortest and the safest access to the nodule.
- •
Biopsy tray setup: the equipment required for a thyroid nodule FNA is simple. The biopsy tray we use includes the following items ( Fig. 2 ):
- ○
Disposable 10-mL plastic syringes
- ○
Disposable 25-gauge and 22-gauge needles, 3.8 cm (1.5 inches) long
- ○
Glass slides for preparation of cytology smears
- ○
Sterile gauze pads
- ○
Lidocaine (1% lidocaine buffered with 8.4% sodium bicarbonate)
- ○
ChloraPrep: 2% weight per volume chlorhexidine gluconate and 70% volume per volume isopropyl alcohol
- ○
Koplin jar with alcohol
- ○
Sterile transducer cover
- ○
Sterile drape with a hole

Fig. 2
Biopsy tray setup. A simple setup consists of 10-mL plastics syringes, disposable 25-gauge and 22-gauge needles, glass slides for cytology smears, sterile gauze pads, sterile drape with the hole, ChloraPrep, and transducer cover.
- ○
- •
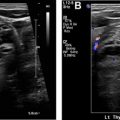
Procedure technique: before beginning the procedure, a time-out is performed to verify the patient’s name and date of birth and to confirm the biopsy target as per the requisition. Once the ultrasonography transducer is selected, it is wiped with an alcohol-based swab, and a short sterile transducer cover is used for covering the transducer and maintaining sterility during the procedure ( Fig. 3 ). After disinfecting the skin with an antiseptic agent (we use ChloraPrep), a sterile drape is used to cover the neck while centering the hole in the drape over the planned biopsy trajectory mark. A small amount of local anesthesia is injected at the point of entry, and a wheal is created on the dermis. This stage is followed by a deeper infiltration of the soft tissues to the thyroid capsule ( Fig. 4 ). Although the wheal on the skin is performed without the use of the transducer, the deeper anesthesia of the subcutaneous fat planes and muscles is obtained by directing the needle under ultrasonography guidance.At our institution, we use a 25-gauge needle to sample the nodule. Most of the time, the scraping effect of the beveled needle edge combined with the repeated excursions in the tissue is enough to procure the cells needed for the diagnosis. In some instances when the nodule is more fibrotic, a syringe may be attached to the needle to apply suction during the procedure. Rarely, a larger-gauge needle, such as 22 to 23 gauge, may be used to obtain adequate material. Very rarely, a longer needle (a spinal needle) may be used if the target lesion is deep in the neck or the patient has a bulky large neck.The visualization of the needle should be oriented in the long-axis plane because the needle is visualized in its entire trajectory ( Fig. 5 ). It makes for good habit to track the needle from its insertion into the skin, its advancement through the soft tissues, and its ultimate entry into the target nodule. In rare situations, a short-axis approach may be performed, wherein the needle is inserted in the short axis along the middle of the transducer. In this instance, only the tip of the needle is visualized and the entire trajectory path is not visualized ( Fig. 6 ). The latter technique has found increased use in interventional radiology for vascular access and is mentioned in this article as an alternate methodology. The authors strongly recommend using the former technique for neck biopsies (ie, the method that visualizes the needle in the long-axis plane in its entire trajectory; see Fig. 5 ).The process of traversing the nodule with the needle is a skill that has a learning curve. Slow, deliberate excursions of the needle within the nodule are observed in real time. Some experts perform rotation of the needle as the sample is being taken. Conceivably, the rotation of the sharp beveled needle helps scrape more relevant material for cytology. Cystic or necrotic areas within the nodule are best avoided during the FNA. The advantages of using only the needle for procuring the cellular material are the flexibility in manipulation of the needle by holding it from the hub and easier visualization of the appearance of aspirate in the hub as an end point. Attaching a 5-mL or 10-mL syringe to the needle can also help to better control the needle and the ejection of the material after sampling. Adopting 1 of the 2 methods (syringe aspiration or not) is also a matter of personal preference and does not matter as long as the eventual outcome is the procurement of a high-yield aspirate.It is important to keep an eye on the hub of the needle as you perform the excursions. When we see a drop of blood in the hub of the needle, we do not perform further excursions. Excessive blood within the hub of the needle only increases the contamination of the specimen with red blood cells without increasing cellular material. Once the material is obtained, we smear the material on a slide. In addition to the smear, we also flush the needle with Cytolyte solution so that a cell block (cells coalesced using a centrifuge) is available for the cytopathologist for further evaluation if needed. We try to do at least 5 passes to obtain adequate material. In our practice, the smear is prepared by the radiologist performing the procedure. In other practices, the smear may be prepared by a cytology technician or an on-site pathologist. In some instances, the on-site pathologist confirms the presence of adequate cellular material after the first or second pass, thus limiting the total number of passes needed. The slides are sent to the laboratory, usually placed in alcohol; in some cases, air-dried slides can be of diagnostic value.