Neonatal Neurosonography
Sonography provides a cost-effective, readily available method to image the infant brain. It is especially valuable because it can be performed at the bedside of premature or critically ill infants, keeping the infant within the protective environment of the neonatal intensive care unit. US is particularly accurate in the detection and follow-up of intracranial hemorrhage and is used to screen for a wide variety of congenital brain anomalies and for complications of central nervous system infection [1].
Spinal sonography is used to demonstrate the anatomy of the spinal canal and the position of the conus medullaris in infants at risk for a tethered spinal cord.
Cranial Sonography
Imaging Technique
The anterior fontanelle provides an excellent sonographic window to image the infant brain. High-frequency 7.5-MHz sector or curved array transducers are utilized to examine the brain in angled sagittal and coronal planes. From the anterior fontanelle, the transducer is swept from the frontal to the occipital lobes in coronal orientation to obtain symmetrical images of the cerebral hemispheres and lateral ventricles. Care must be taken to orient the transducer properly to display the right side of the infant brain on the left side of the image. The transducer is turned 90 degrees for the sagittal plane. A midline sagittal view demonstrates the third ventricle, brain stem, and posterior fossa. The transducer is angled right and left from its midline sagittal position to examine each hemisphere and lateral ventricle in detail. The posterior fontanelle, the foramen magnum, and the thin squamous portion of the temporal bone may be utilized to provide supplementary images whenever indicated [2,3]. Lower-frequency transducers (3.5-5.0 MHz) may be needed to improve penetration in older and larger infants. Representative views of the cerebral hemispheres, lateral ventricles, third ventricle, choroid plexus, caudothalamic groove, corpus callosum, cavum septum pellucidum/vergae, fourth ventricle, and vermis of the cerebellum are documented [4]. US examination can routinely be performed up to approximately 12-14 months of age when the fontanelle becomes too small to serve as an effective window.
Anatomy
Basic Infant Brain Anatomy
Several concepts of infant brain anatomy must be kept in mind when performing and interpreting sonography of the neonatal brain. The ventricles can be conceptualized as the easily identified “skeleton” of the brain. Their complex shape must be recognized in each plane of section. Surrounding structures are then identified with respect to their location relative to the ventricles. The lateral ventricles form the framework of each cerebral hemisphere. Each lateral ventricle has a frontal horn, body, atrium, occipital horn, and temporal horn. The lateral ventricles are commonly (in up to 70% of individuals) mildly asymmetric in size. Normal lateral ventricles may be tiny slits or form angulated or comma-shaped lucencies on coronal images [5]. The atrium or trigone is the junction of the temporal and occipital horns with the body of the lateral ventricle. The choroid plexus functions as the site of production of cerebrospinal fluid (CSF) and forms a prominent echogenic structure that lines the temporal horn, atrium, and body of each lateral ventricle. No choroid plexus is present in the frontal horns or occipital horns. This is most important to note because acute hemorrhage has the same echogenicity as the choroid plexus and is recognized primarily by location. Echogenic material in the anterior or occipital horns is hemorrhage, not choroid plexus. The choroid plexus is biggest in the atrium of each lateral ventricle. This prominent blob of choroid plexus in the atrium is called the glomus. The choroid plexus extends forward from the glomus to pass through the foramen of Monro and then reflects posteriorly along the roof of the third ventricle. The anterior-most portion of the choroid plexus in the roof of the third ventricle serves as a marker for the location of the foremen of Monro.
The germinal matrix is a group of loosely organized proliferating cells that give rise to the neurons of the cerebral cortex during embryologic development. The germinal matrix is exceptionally vascular with a network of thin fragile capillaries highly susceptible to injury by hypoxia. In early gestation, the germinal matrix lines the wall of the entire ventricular system, lying just beneath the ependyma, the thin membranous lining of the ventricular system. After 12 weeks gestation, the germinal matrix begins to regress. By 24 weeks, only the germinal matrix over the caudate nucleus persists. By full term at 40 weeks, the germinal matrix no longer exists. Thus hemorrhage of the germinal matrix is a disease of premature infants. It originates in the residual germinal matrix that overlies the caudate nucleus in the frontal horns of the lateral ventricles. The normal germinal matrix is not visualized by US.
A prominent fluid-filled structure is seen in the midline of the developing brain. This cystic structure is solitary and continuous, but for reasons unknown to me, it is given two unhandy names [6]. The anterior portion is called the cavum septum pellucidum whereas the posterior portion is called the cavum vergae. The foramen of Monro marks the divide between the two names. This structure involutes from back to front during fetal life and infancy. At full term, only the cavum septum pellucidum exists in most infants. This structure normally closes completely by 3-6 months of life. However, in approximately 5% of adults it persists as a residual fetal structure. The cavum septum pellucidum/vergae has been mistaken for hydrocephaly.
In premature infants, the surface of the cerebral hemispheres is smooth and flat [7]. With development between the equivalent of 24 and 40 weeks gestation, the sulci deepen, bend, and branch to form the prominent normal gyral pattern of full-term infants.
Although cisterns are by definition CSF-filled spaces around the brain, on US the cisterns are often echogenic rather than echolucent. The echogenicity is produced by reflection from folds of meninges that float in the fluid of the cisterns.
Coronal Plane Anatomy
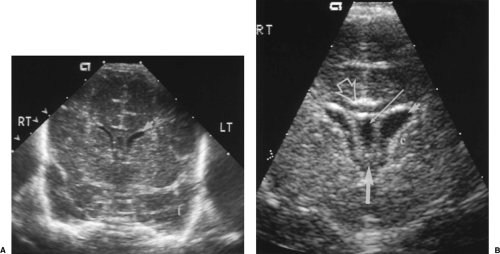
In coronal plane, the most anterior section is obtained anterior to the frontal horns. This image documents the frontal lobes extending over the orbits (Fig. 10.1).
The next plane posterior documents the frontal horns, which appear as paired slit-like or comma-shaped anechoic structures (Fig. 10.2). The caudate nuclei form the angled
floors of the frontal horns and must be inspected in detail to detect small echogenic hemorrhages of the germinal matrix. The cavum septum pellucidum is the anechoic fluid-filled structure in the midline. The hypoechoic band extending between the cerebral hemispheres just above the cavum septum pellucidum is the corpus callosum [8]. The midline
sagittal fissure extends to the level of the corpus callosum. Anterior cerebral arteries pulsate and are visible with color Doppler in the sagittal fissure. The tips of the temporal lobes are seen inferiorly and laterally.
floors of the frontal horns and must be inspected in detail to detect small echogenic hemorrhages of the germinal matrix. The cavum septum pellucidum is the anechoic fluid-filled structure in the midline. The hypoechoic band extending between the cerebral hemispheres just above the cavum septum pellucidum is the corpus callosum [8]. The midline
sagittal fissure extends to the level of the corpus callosum. Anterior cerebral arteries pulsate and are visible with color Doppler in the sagittal fissure. The tips of the temporal lobes are seen inferiorly and laterally.
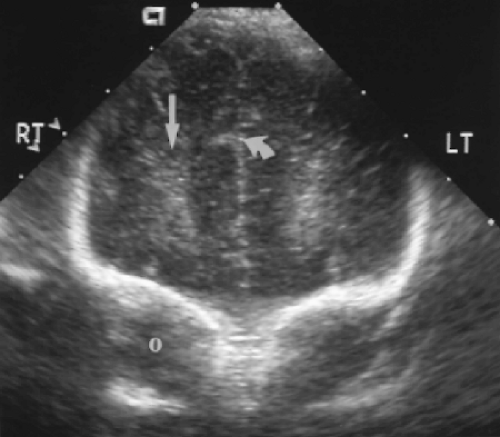
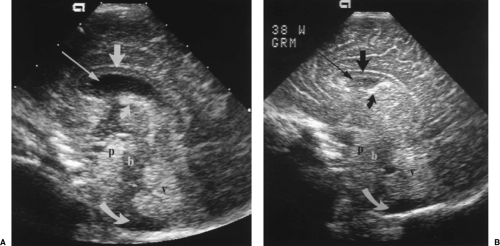
Moving posteriorly to a direct coronal plane, the echogenic choroid plexus is visualized in the midline just below the cavum septum pellucidum (Fig. 10.3). The choroid plexus is normally present in both lateral ventricles in the same anatomic planes where the choroid is visualized in the roof of the third ventricle, but not in the frontal horns more anteriorly. The third ventricle is slit-like and may be seen as a linear lucency between the oval globes of the thalami. Commonly the normal third ventricle is not seen as a discrete structure. Portions of the hypoechoic brainstem are seen in the midline below the third ventricle and thalami.
A posteriorly angled coronal plane demonstrates the prominent echogenic glomus of the choroid plexus in atria of the lateral ventricles (Fig. 10.4). The glomus swings dependently toward the downside of the baby’s head, commonly revealing a gap between the wall of the lateral ventricle and the choroid plexus. The tentorium may be seen as an echogenic wall between the cerebrum and cerebellum.
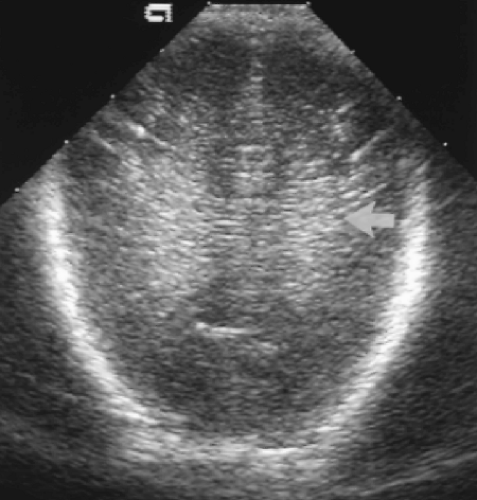
Further posterior angulation of the transducer produces an image of the occipital lobes above the level of the occipital horns (Fig. 10.5). Vague symmetric echogenicity should be evident bilaterally. The echogenicity is produced by sound reflection from periventricular white matter tracts. The acute hemorrhagic stage of periventricular leukomalacia (PVL) produces asymmetric or increased echogenicity in this area. Cystic change is easily seen in this plane during the later cystic stage of PVL.
Sagittal Plane Anatomy
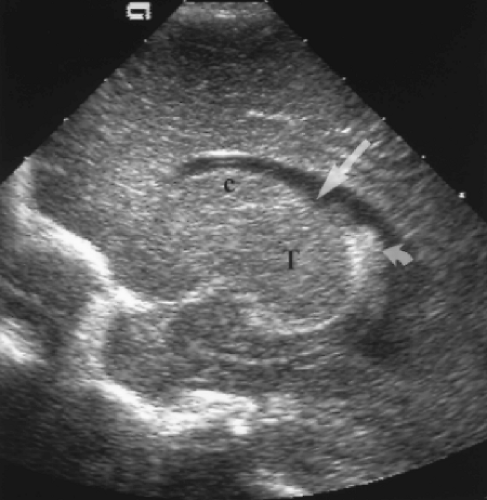
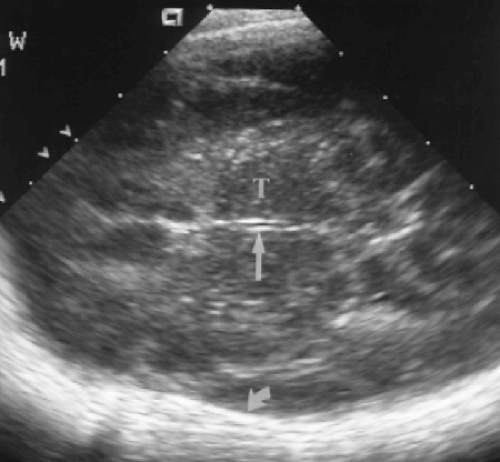
A direct midline sagittal image demonstrates the corpus callosum as a well-defined, curving hypoechoic band above the cystic cavum septum pellucidum/vergae (Fig. 10.6) [8]. The anterior genu and posterior splenium are normally slightly thicker than the trunk of the corpus callosum. The entire corpus callosum should be visualized to avoid missing partial agenesis. Immediately inferior to the cavum septum pellucidum is the curving echogenic band of the choroid plexus in the roof of the third ventricle. The third ventricle is normally slit-like and is not distinctly visualized. The midline cerebellar vermis is seen as a rounded echogenic mass in the posterior fossa. The fourth ventricle is visualized as a
triangular lucency at the mid-base of the echogenic vermis [9]. Anterior to the vermis is the hypoechoic and poorly defined brainstem. The pons is more echogenic than the remainder of the brainstem. Special note should always be made of the presence or absence of the cisterna magna inferior to the vermis. Obliteration of the cisterna magna is seen with Chiari malformation.
triangular lucency at the mid-base of the echogenic vermis [9]. Anterior to the vermis is the hypoechoic and poorly defined brainstem. The pons is more echogenic than the remainder of the brainstem. Special note should always be made of the presence or absence of the cisterna magna inferior to the vermis. Obliteration of the cisterna magna is seen with Chiari malformation.
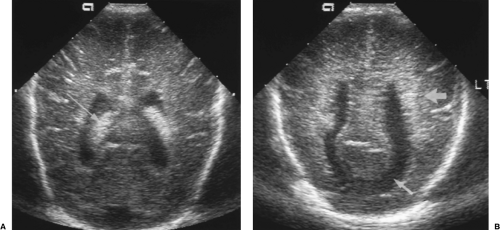
Approximately 10 degrees of lateral angulation to each side will demonstrate the lateral ventricles (Fig. 10.7). Note that the frontal horns are slightly closer together than the atria of the lateral ventricles. To show the long axis of the ventricles, the posterior aspect of the transducer is angled slightly more laterally than the anterior portion. The important landmarks here are the round shape of the thalamus and the tongue shape of the caudate nucleus. These two structures are nearly isoechoic but are separated by a slight V-shaped defect and echogenic line called the caudothalamic groove. The caudothalamic groove marks the location of the foramen of Monro and the anterior-most location of the choroid plexus [10]. Echogenicity in the caudate nucleus anterior to the caudothalamic groove indicates hemorrhage in the germinal matrix. The lateral ventricle enlarges posteriorly and contains the glomus of the choroid plexus. The glomus is commonly lobulated in contour. The occipital horns may be seen further posteriorly. Their size is quite variable and they are commonly asymmetric. Remember no choroid plexus is present in the occipital horns, so echogenicity in the occipital horns usually represents intraventricular blood clot.
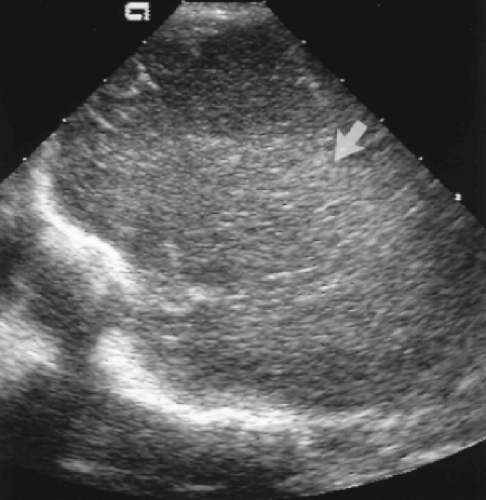
Further lateral angulation will demonstrate the ill-defined normal echogenicity of the periventricular white matter tracts (Fig. 10.8). Asymmetric or increased echogenicity suggests the hemorrhagic stage of PVL. This laterally angled image plane is best for demonstrating the cystic change of PVL as well.
Axial Plane Anatomy
Axial plane images are a useful addition to routine examination. The squamous portion of the temporal bone is thin enough in infants to allow adequate US penetration. Two image planes are most useful.
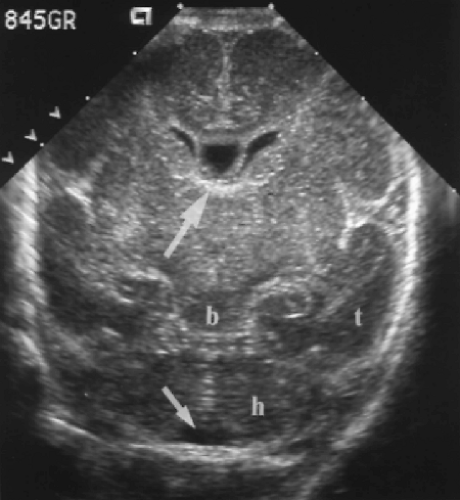
A mid-thalamic plane, similar to the plane used to measure biparietal diameter in the fetus, demonstrates the third ventricle and thalami (Fig. 10.9). The third ventricle is normally a narrow slit. In axial plane the sound beam is perpendicular to the lateral walls of the third ventricle and shows the size of the ventricle better than the coronal or sagittal plane. Axial plane views also demonstrate the lateral interface between brain and cranium and are useful for detection of extra-axial fluid collections and abnormalities of the cerebral cortex.
Just inferior to the plane of the third ventricle is the plane of the suprasellar cistern (Fig. 10.10). The cistern has the appearance of a 5-pointed echogenic star. The hypothalamus is seen as an oval structure centered in the cistern. The arteries of the circle of Willis are clearly visualized as pulsating vessels encircling the cistern on real-time US. This is an excellent plane for Doppler evaluation of these vessels. Posterior to the cistern is the heart-shaped structure of the cerebral peduncles. In the midline posterior aspect of the peduncles courses the cerebellar aqueduct, seen as an echogenic dot with or without a central lucency.
Hypoxic Brain Injury
Hypoxia is a common event in the sick neonate, especially in the premature infant with underdeveloped lungs. Hypoxia may result in two basic types of infant brain injuries: germinal matrix hemorrhage (GMH) and PVL [11]. The prevalence of GMH in infants born before 32 weeks gestation or with birth weight less than 1500 g is 30-55%. The prevalence of PVL in these infants is 10-40%.
It is important to note that the arterial watershed areas are different in the infant brain than in the adult or older child. In the premature infant, the watershed areas most susceptible to hypoxic injury are in the regions of the periventricular white matter. Autoregulation of cerebral blood pressure is commonly lacking in the neonate, making the infant more susceptible to hypoxic injury and hemorrhage associated with hypotension or hypertension.
Germinal Matrix Hemorrhage
GMH is a disease confined to premature infants, because the germinal matrix resorbs and is no longer present at full term. GMH is also commonly called subependymal hemorrhage
because of the subependymal location of the germinal matrix, or is called intraventricular hemorrhage because extension of hemorrhage into the ventricles is common. The Papile classification of GMH is commonly utilized (Table 10.1) [12]. More severe grades of hemorrhage are predictive of worse neurological prognosis. GMH may result in cerebral palsy, developmental delays, and learning disabilities (see Fig. 10.14) [13]. Hemorrhages occur initially in the subependymal caudate nucleus in the frontal horn and then extend elsewhere in the brain [14].
because of the subependymal location of the germinal matrix, or is called intraventricular hemorrhage because extension of hemorrhage into the ventricles is common. The Papile classification of GMH is commonly utilized (Table 10.1) [12]. More severe grades of hemorrhage are predictive of worse neurological prognosis. GMH may result in cerebral palsy, developmental delays, and learning disabilities (see Fig. 10.14) [13]. Hemorrhages occur initially in the subependymal caudate nucleus in the frontal horn and then extend elsewhere in the brain [14].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree