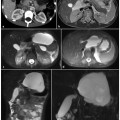
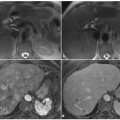
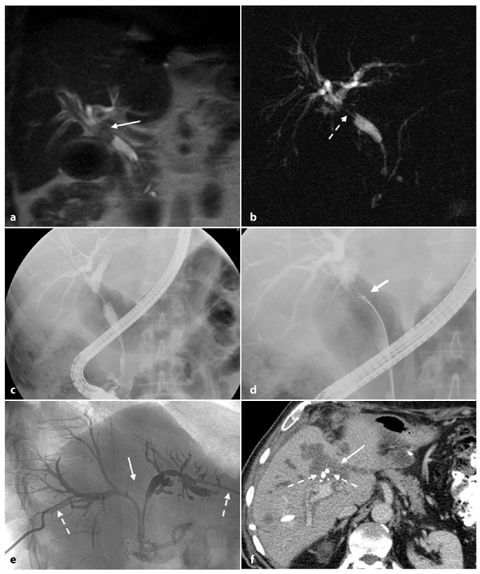
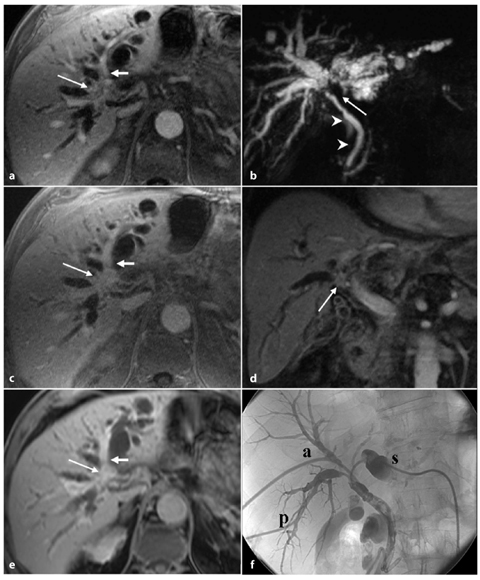
Fig. 5.1
Peripheral cholangiocarcinoma. Axial magnetic resonance (MR) T1-weighted images during the arterial (a) and portal venous (b) phases show a nodular lesion in the fourth segment with heterogeneous and peripheral enhancement of the contrast medium in the early phase and slow accumulation during the portal venous phase (arrow). c The coronal post-contrast MR T1-weighted image shows the origin of the lesion (arrow) in the parietal thickening of a bile duct responsible for dilatation of the upstream biliary branches (arrowheads). d MR cholangiopancreatography confirms exclusive dilatation of the bile ducts in the paramedian segments of right hepatic lobe (arrowheads), and this comes to an abrupt halt at the right secondary biliary bifurcation (arrow). e Axial contrast-enhanced computed tomography section after a surgical procedure (mesohepatectomy extended to caudate lobe) shows multiple metallic clips (short arrows) and patency of the left portal branch (dashed arrow). f Post-operative cholangiography performed through two biliary drainages (open arrow) shows the normal caliber of the bile ducts for the right posterior-lateral sector (p) and the intrahepatic biliary ducts of the left lobe (s)
For intrahepatic cholangiocarcinoma, prognostic factors confirmed on multivariate analysis include tumor number and differentiation, lymph node metastases and vascular invasion [9]. While US can usually detect only focal duct dilatation, CT and magnetic resonance imaging (MRI) are comparable in the detection and correct diagnosis of the tumor. CT is the preferable technique for the assessment of vascular encasement [10]. Traditional direct cholangiography, now replaced by MRCP, is the technique of choice for evaluating the extent of biliary disease, showing stricture, segmental or lobar bile duct dilatation, and filling defects. MRCP, especially if it is included in a complete study of the upper abdomen, with axial T1-weighted and T2-weighted imaging, can allow detection, characterization and staging of the lesion, giving the clinician all the information necessary for adequate treatment planning [11]. In patients selected for major hepatic resection, portal vein embolization, via either the percutaneous transhepatic or the transileocolic route, has been proposed as a useful procedure in pre-operative treatment for patients whose liver remnants will be too small to allow resection. In this way, selective hypertrophy of the healthy portion of the liver can be achieved [12]. CT and MRI are useful tools to depict hepatic segmentation and portal venous anatomy before performing this procedure.
5.2.2 Hilar Cholangiocarcinoma: Klatskin Tumor
Hilar cholangiocarcinoma accounts for 25% of all cholangiocarcinomas. It arises from the right or left hepatic ducts, and can involve the bifurcation of the common hepatic duct. The middle and distal portions of the common hepatic duct are affected in about 17% and 18% of cases, respectively, while there is diffuse cancerous involvement in about 7% of cases [7]. Since this type of tumor causes localized biliary duct stricture, it is discovered early, usually when it is very small, because of the presence of jaundice or cholangitis. Typical spread occurs by local extension from the biliary tree invading the liver. Perineural invasion and periductal spread, which are frequent in hilar cholangiocarcinoma, are usually underestimated and they represent a cause of treatment failure. Surgery is the only therapeutic option, and the role of diagnostic imaging in this type of tumor is to assess tumor resectability by identifying the level of bile duct obstruction, and the presence or absence of vascular invasion, satellite nodules, intrahepatic tumor spread and lymph node involvement [13].
Criteria for unresectability include the following: evidence of bilateral extension to the segmental branches of the intrahepatic bile ducts; infiltration of the main portal vein of one lobe of the liver, combined with involvement of the hepatic artery of the other lobe; a combination of vascular involvement in one side of the liver with extensive bile duct involvement of the contralateral side; hepatic or nodal metastases [14].
Sonography is usually the initial imaging study in patients with jaundice: it is accurate in visualizing the biliary obstruction at the level of the hepatic hilus, but not equally as effective in demonstrating the obstructing lesion. Overall, sonography detects about 21–45% of these tumors. Although it is difficult to diagnose hilar cholangiocarcinoma with conventional CT because of its small size, spiral or multidetector CT allows more effective evaluation of these small lesions, and better demonstrates the status of the hepatic arterial or portal venous circulation. Although the accuracy of CT in establishing tumor resectability is about 60%, invasion of the intrahepatic bile ducts is usually underestimated in 13% of patients and overestimated in 4% [15].
MRI in combination with MRCP can enable direct visualization of hilar cholangiocarcinoma and determine resectability [14]. The morphology of bile duct stricture detectable on MRCP closely reflects the gross morphological changes occurring along the biliary ductal walls. MRCP can visualize intraluminal tumor extent and characteristics of bile duct stricture. A recent study demonstrated that accuracy of MRCP and endoscopic retrograde cholangiopancreatography (ERCP) in assessing the level and features of bile duct obstruction are similar, while accuracy of MRCP is superior in determining suprahilar tumor extension [14]. Another study comparing the accuracy of MRCP and ERCP in detecting the cause of perihilar biliary obstruction revealed superior sensitivity of MRCP in describing the cause and depicting the anatomical extent of the jaundice, especially in cases associated with tight biliary stenosis and long segmental biliary stricture [16, 17]. MRCP, in contrast to ERCP, can depict bile ducts both proximal and distal to the obstruction. Furthermore, combined use of MRCP and dynamic MRI can display the overall extent of biliary tree involvement, describing vascular and liver parenchyma invasion. Perineural spread, correlated with delayed enhancement along the bile ducts, can also be identified with MRI.
For pre-operative assessment of resectability of hilar cholangiocarcinoma, however, several types of invasive imaging, such as cholangiography and angiography, are sometimes required. Cholangiography, through a retrograde endoscopic or percutaneous transhepatic (PTC) approach, can provide the most accurate anatomical information pertaining to which segmental branches are involved, but it is an invasive technique. For this reason and because of the availability of non-invasive imaging techniques with the same diagnostic accuracy such as MRCP, ERCP and PTC are today considered to be therapeutic tools only. Furthermore, since bile duct visualization in ERCP depends on the duct opacification with injected contrast medium, duct opacification may be limited because of technical demands of duct cannulation and gravitational factors, as occurs when the contrast medium is heavier than the bile and it does not adequately fill the proximal ducts. Previous abdominal surgery, such as hepatojejunostomy or gastrojejunostomy, can be an obstacle for ERCP, but not for MRCP [18].
Differential diagnosis must be made first with other diseases that can cause hilar obstruction indistinguishable from hilar cholangiocarcinoma; these include: metastases to periportal lymph nodes, gallbladder cancer invading the hepatoduodenal ligament, lymphadenopathy due to other inflammation, and idiopathic benign focal stricture of the bile duct (Fig. 5.2).
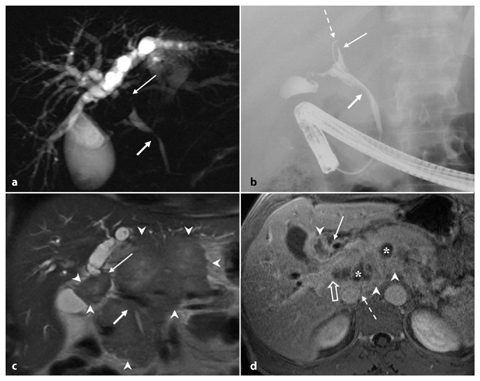

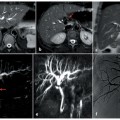
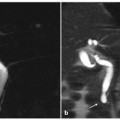
Fig. 5.2
Hilar lymphoadenomegaly. a At MR cholangiopancreatography, the common hepatic duct is occluded (long arrow) and the intrahepatic bile ducts are markedly dilated; below the common bile duct there is narrowing of the caliber in the middle third (short arrow). b Endoscopic retrograde cholangiopancreatography (ERCP) showing a guide wire that reaches the biliary confluence (dashed arrow), and opacification of extrahepatic bile ducts; the ERCP findings are comparable with those observed at MRCP. c Coronal magnetic resonance (MR) T2-weighted half-Fourier acquisition single-shot turbo-spin-echo (HASTE) section showing the etiology of the occlusion of the common hepatic duct (long arrow) and of the stenosis of the common bile duct (short arrow): a bulky solid mass, hypointense, located in the hilar region, portacaval space and along the hepatoduodenal ligament (arrowheads), surrounding and compressing the extrahepatic bile ducts. d Axial MR T1-weighted section in the venous phase showing the newly formed tissue (arrowheads) appearing as a nodal agglomeration, with inner necrotic areas (asterisks), that includes the portal vein (open arrow), coming into contact with the inferior vena cava without infiltrating it (dashed arrow) and occluding the common hepatic duct (arrow)
According to the Bismuth-Corlette classification, hilar stenosis can be classified in four groups (Fig. 5.3).
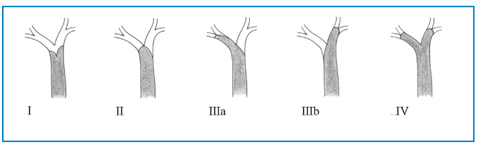
Type I: tumors involving the common hepatic duct proximal to the primary hepatic confluence (Fig. 5.4).
Type II: tumors involving the hepatic duct bifurcation and both hepatic ducts (Fig. 5.5).
Type IIIa: tumors involving the right secondary confluence (Fig. 5.6).
Type IIIb: tumors involving the left secondary confluence (Fig. 5.7).
Type IV: tumors involving both secondary confluences (Fig. 5.8).

Fig. 5.3
Bismuth-Corlette classification of hilar cholangiocarcinoma

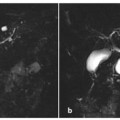
Fig. 5.4
Type I Klatskin tumor. Magnetic resonance (MR) imaging on coronal T2-weighted half-Fourier acquisition single-shot turbo-spin-echo (HASTE) (a) and MR cholangiopancreatography (b) showing intrahepatic bile ducts that appear moderately dilated up to the common hepatic duct where an endoluminal formation (arrow, a) is recognizable. Internal-external biliary drainage is shown by the dashed arrow. c, d The framework shown in the MR imaging is completely comparable to endoscopic retrograde cholangiopancreatography shown here, and during which sampling of endoluminal tissue is performed by biopsy forceps at the level of the common hepatic duct (short arrow, d). At 6 months after resection of the common bile duct, cholecystectomy and biliary-jejunal anastomosis, the patient develops jaundice. e During placement of two external-internal biliary drainage tubes for both sides (dashed arrows), the cholangiography examination documents the dilatation of intrahepatic bile ducts in relation to the extensive occlusion of the confluence (arrow). f Axial contrast-enhanced computed tomography section showing heterogeneously hypodense area at the confluence of the bile ducts (arrow) compatible with recurrent disease. The two external-internal biliary drainage tubes are recognizable (dashed arrows)

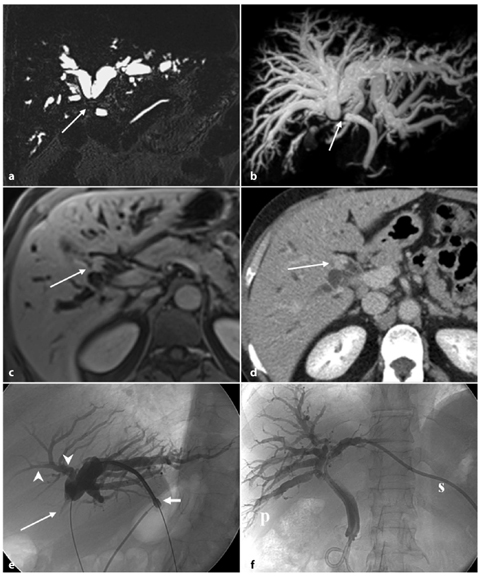
Fig. 5.5
Type II Klatskin tumor. Three-dimensional MR cholangiopancreatography in thinner (a) and thicker section (b) showing the occlusion of the biliary confluence (arrow), which is responsible for the widespread dilatation of the entire biliary tree. Axial contrast-enhanced late phase magnetic resonance (c) and computed tomography (d) sections showing thickening of the walls of biliary confluence responsible for occlusion of the lumen (arrow); the newly formed tissue shows infiltrative growth and accumulation of contrast medium in late phase in both methods. e Cholangiography of the left biliary hemisystem through percutaneous access in paraxiphoid (short arrow) confirming the abrupt, tight stenosis of the confluence (long arrow); the visualization of the bile ducts for the fourth hepatic segment (arrowheads) is needed to assess that the secondary division of the left hepatic duct is not involved by the disease, in planning a right hemi-hepatectomy. f The patient undergoes placement of two internal-external biliary drainage tubes, one for the right posterior-lateral segment (p) and other for the third hepatic segment (s) to reduce jaundice, and prevent cholangitis, while waiting for a surgical procedure

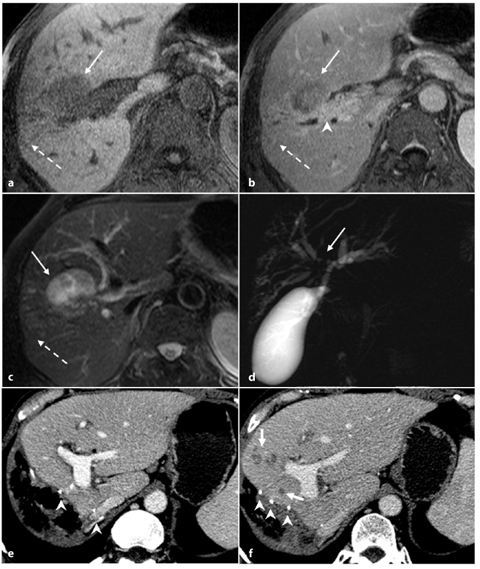
Fig. 5.6
Type IIIA Klatskin tumor. a, b Axial magnetic resonance T1-weighted images showing a hypointense focal liver lesion (arrows) with progressive retention of contrast agent (b). c T2-weighted image showing the neoplasm appearing mildly hyperintense. The lesion has its epicenter in the right hepatic duct, which presents thickened walls with delayed retention of contrast medium (arrowhead, b); glissonian retraction coexists (dashed arrows). d MR cholangiopancreatography in which dilatation of intrahepatic bile ducts is recognized because of stenosis of the primary biliary confluence (arrow). e Axial contrast-enhanced computed tomography (CT) section after intervention of hemi-hepatectomy, showing multiple clips (arrowheads, e, f) recognizable along the shearing of resection. f Axial contrast- enhanced CT section in the same craniocaudal position (as shown in e) 1 year later: some small hypodense hepatic lesions during the portal venous phase (short arrows) have appeared marginally to the shearing of resection, compatible with recurrent disease

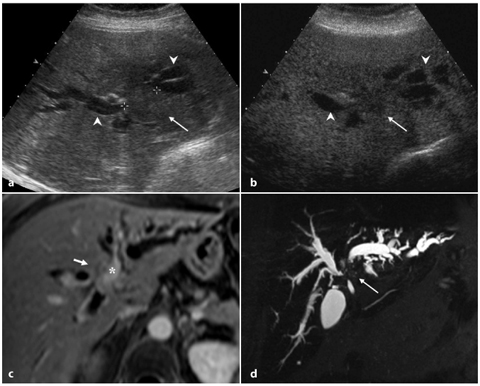
Fig. 5.7
Type IIIB Klatskin tumor. a Ultrasound showing a slightly hypoechoic nodule (arrow) recognizable at the biliary confluence, with consequent dilatation upstream of the intrahepatic bile ducts of both hemi-systems (arrowheads). b Contrast-enhanced ultrasound (15 min after injection) showing the lesion (arrow) with no contrast enhancement because of the intravascular nature of the contrast medium. c Post-contrast axial T1-weighted magnetic resonance image showing weak enhancement of bile duct, which appears thickened (short arrow) and in close proximity to the left portal branch (asterisk). d MR cholangiopancreatography showing diffuse dilatation of the entire biliary tree that is abruptly interrupted at the confluence (arrow)

Fig. 5.8
Type IV Klatskin tumor. Axial magnetic resonance imaging (MRI) sections in early (a), venous (c) and delayed (e) contrast-enhanced phase show dilatation of the intrahepatic bile ducts of both lobes because of biliary stenosis at the primary confluence (long arrow), in which we can observe in the venous phase a nodular lesion, with slight enhancement in early phase and increased enhancement during the late phase, which causes a reduction of the left portal branch caliber (short arrow). b MR cholangiopancreatography confirms the dilatation of the intrahepatic biliary tree up to the primary confluence (arrow). Downstream, the size of the common bile duct up to the papilla is regular (arrowheads). d A coronal post-contrast T1-weighted MRI during the portal venous phase shows the newly formed solid tissue (arrow) at the biliary confluence. The palliation of jaundice and cholangitis requires the placement of three external-internal biliary drainage (cholangiography, f): one for the left hemi-system (s), one for posterolateral sectors (p) and one for paramedian sectors (a) of the right hemi-system, indicating a disease extent at second-order biliary bifurcation
5.2.3 Distal Extrahepatic Cholangiocarcinoma
Extrahepatic cholangiocarcinoma arises between the common bile duct and the ampulla of Vater. In 50–75% of reported cases it occurs in the upper third of the duct, including the hepatic hilum, in 10–30% it occurs in the middle third, and in 10–20% it occurs in the lower third [19].
Because of the site, the tumor usually causes biliary obstruction and jaundice and it manifests early when the mass is small (Fig. 5.9). Bile ducts proximal to the tumor are usually enlarged, while at the level of the tumor they are completely obstructed, and distal to the mass they are of normal diameter. Involvement of the periductal lymphatics, nerves and perineural tissue is frequent.
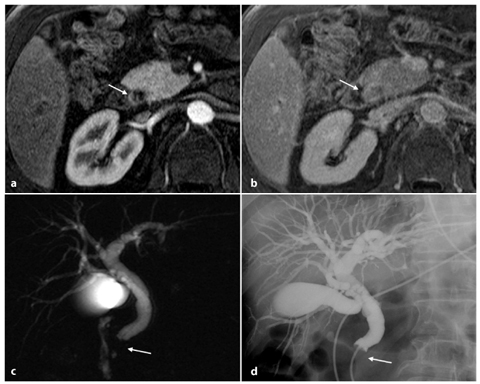

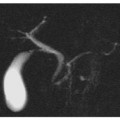
Fig. 5.9
Cholangiocarcinoma of the terminal common bile duct. Axial contrast-enhanced MR sections in early (a) and late (b) phase in the same craniocaudal position show a late impregnation of endoluminal contrast medium in the prepapillary common bile duct region (arrow). c MR cholangiopancreatography shows a widespread dilatation of the entire biliary tree visible to the level of the terminal common bile duct, which is occluded (arrow). d The findings described in c are completely comparable to those of endoscopic retrograde cholangiopancreatography examination
Because of the difficulty of locating lesions in the early stages of growth, and their poor responses to therapy, extrahepatic cholangiocarcinoma is a challenging pathology in terms of diagnosis, treatment and management.
Since surgical resection of all detectable tumor is the only therapy that improves 5-year survival rate (patients with unresectable cholangiocarcinoma typically die within 6–18 months from the date of diagnosis), the role of diagnostic imaging is extremely important in terms of obtaining tumor-free surgical margins. The major determinants of resectability to consider are: extent of tumor within the biliary tree, vascular invasion, hepatic lobar atrophy and metastatic disease. Hepatic lobe hemiatrophy is usually the result of portal vein involvement or a longstanding biliary obstruction. Involvement of a segment of portal vein shorter than 2 cm can allow tumor resection with vein reconstruction. Otherwise, the presence of lymph node metastases at the N2 level, or at the celiac, periportal or superior mesenteric level, is a contraindication to surgery because it is usually associated with advanced disease [20].
Specifically in the field of diagnostic imaging, no single method that is capable of detecting and accurately localizing cholangiocarcinoma has been introduced, and comprehensive and rigorous approaches using invasive and non-invasive modalities are needed to enable an accurate pre-surgical evaluation of this disease.
Although ultrasonography is the first diagnostic imaging procedure used in cases of extrahepatic cholangiocarcinoma, it can usually detect only common indirect signs of the tumor, such as an abrupt change in ductal diameter with ductal dilatation above it. Contrast-enhanced CT can assess, with good accuracy, the level of obstruction, the vascular involvement, liver atrophy and enlarged lymph nodes, and metastases. It has been demonstrated that CT with axial and coronal images tends to underestimate the longitudinal tumor extent [21]. MRI can evaluate the resectability of biliary duct cancer with more advantages. Furthermore, with MRI it is possible to comprehensively evaluate biliary duct cancer because of the variable tissue contrasts involved. In most cases, cholangiocarcinoma shows low intensity on T2 contrast images because of its rich collagenous fiber content. T1-weighted images are informative when used with fat-saturation pulses and an extracellular contrast agent, such as a gadolinium chelate. A bile duct wall that is thickened by a cholangiocarcinoma shows strong enhancement during the equilibrium phase of MRI. This enhancement may be explained in the same manner as enhancement by an iodinated contrast medium on CT. In addition, MRCP can show the biliary duct lumen, assessing it both upstream of a tumor, where is often dilated, and downstream of the tumor in the same examination [20]. The sensitivity of MRCP in defining stenoses and the level of obstruction has been reported to be in the order of 86–100% [22]. The biliary tree is depicted on both sides of a stricture, thereby defining the stricture length, its proximal extent, and its anatomical relations with other intrahepatic bile ducts. In cases of biliary drainage positioning, MRCP may not be of diagnostic value because of biliary tree collapse; in such cases, if is not possible to defer the drainage, a slow and careful injection of a small amount of saline through the drainage tube (up to 20 ml) is advised. ERCP can provide useful information with regard to the level of obstruction, and can show clearly that an obstruction is arising from a bile duct and does not involve the pancreatic duct. Direct cholangiography sometimes fails to accurately depict the longitudinal spread of a tumor because tumor extensions are often submucosal or extramural.
Tumor resectability often depends on the extent of vascular involvement. Angiography has played a major role in documenting the vascular encasement of the portal vein and hepatic artery; however, the technique is no longer compulsory as a pre-operative examination in cholangiocarcinoma. Recent MRI and CT developments are increasingly replacing this methodology.
Endoscopic ultrasonography (EUS) can depict the mass within the bile duct and the possible disruption of the subserosal fat layer. It is a useful technique for assessing the distal extrahepatic biliary tree, the gallbladder, and even the regional lymph nodes; in fact, the accuracy of EUS is higher in distal cholangiocarcinoma and in non-stented bile ducts, while the sensitivity of EUS for detecting nodal metastasis in cholangiocarcinoma is quite low [23].
When an obstruction of the biliary tree is detected, distinguishing between a malignant and benign nature of the cause of the obstruction can provide a diagnostic clue. In addition to extrahepatic cholangiocarcinoma, malignant strictures include pancreatic head carcinoma, duodenal carcinoma, metastatic lymph nodes, metastases from hepatocellular carcinoma and gastric carcinoma. Benign strictures can be caused by portal cavernoma, iatrogenic cholangitis, post-traumatic cholangitis and Mirizzi syndrome. In a recent study comparing ERCP and MRCP in the differentiation of extrahepatic cholangiocarcinoma from benign stricture, irregular and asymmetric stricture margins were more common in cholangiocarcinoma, and smooth and symmetric stricture margins were more common in the benign stricture, while irregular margins and asymmetric narrowing were seen in both benign and malignant strictures. The frequency of appearance of the „double-duct sign” was not significantly different between cholangiocarcinomas and benign strictures. Sensitivity, specificity and accuracy for the differentiation of malignant from benign causes of biliary stricture at MRCP were 81%, 70% and 76%, respectively, and at ERCP they were 74%, 70% and 72%, respectively [24].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree