(1)
Department of Clinical Radiology, Amiri Hospital – Kuwait City, Kuwait City, Kuwait
4.1 Hypertension
4.1.1 Renal Artery Stenosis
4.1.2 Coarctation of the Aorta
4.1.3 Polyarteritis Nodosa
4.1.4 Takayasu Arteritis
4.1.5 Midaortic Syndrome
4.1.6 Preeclampsia
4.1.11 Hypertensive Heart Disease
4.3 Renal Failure
4.1 Hypertension
Hypertension is a disease characterized by an increase in systolic blood pressure >140 mmHg and in diastolic blood pressure >100 mmHg. Hypertension is 90 % primary (without a cause) and 10 % secondary to an organic cause. Radiological modalities are mainly used to detect secondary causes of hypertension.
Secondary causes of hypertension include the following:
Renovascular diseases: atherosclerosis (adults), fibromuscular dysplasia (children), vasculitis (polyarteritis nodosa (PAN) and Takayasu arteritis (TA)), and renal artery aneurysm.
Adrenal causes: pheochromocytoma, primary hyperaldosteronism, and Cushing’s syndrome.
Renal parenchymal diseases: chronic glomerulonephritis, diabetic nephropathy, lupus nephritis, polycystic kidney disease, and page kidney.
Aortic diseases: coarctation of the aorta and midaortic syndrome.
Other causes: brain tumors, congenital AVM, carcinoid tumors, acromegaly, and hypercalcemia.
Renal Artery Stenosis
Renal artery stenosis (RAS) constitutes 1–5 % of patients with hypertension. Atherosclerosis is the commonest cause of renovascular hypertension in adults, while renal artery fibromuscular dysplasia is the most common cause of renovascular hypertension in children. Atherosclerosis RAS often affects the proximal part of the artery, while fibromuscular dysplasia often involves the middle and the distal part in a form of small stenotic and aneurysmal dilatation, giving the so-called beaded appearance on angiography.
RAS is suspected as a cause of hypertension in the following situations:
Hypertension in a patient <30 years of age or a patient >50 years
Hypertension that is resistant to three antihypertensive regimens
Sudden renal functions worsening in a hypertensive patient
Sudden development or worsening of hypertension in any age
Unilateral small kidney
Renal impairment after treatment with angiotensin-converting enzymes (ACE) inhibitors
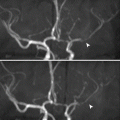
RAS is commonly diagnosed by color-coded duplex scanning by two methods: direct and indirect. The direct method involves measuring the blood velocity directly within the renal artery (Fig. 4.1.1). In contrast, the indirect method involves measuring the blood velocity within the segmental and interlobar intrarenal vessels (Fig. 4.1.2). The indirect method is insensitive for less than 60 % RAS.



Fig. 4.1.1
Color Doppler sonogram of the aorta shows a normal anatomy of the renal arteries (banana peel view) in gray mode in (a) and Duplex-colored mode in (b) taken while the patient is in the lateral decubitus position. The right renal artery is clearly detected in (b) (yellow arrowhead), and the left renal artery is also detected well in this position (blue arrowhead)

Fig. 4.1.2
Color Doppler sonogram of the left kidney demonstrates its vascular anatomy in (b) and arterial waveform detection in (a) to assess the arterial vascular supply as an indirect method for detecting RAS
The resistance index (RI) is the maximal systolic velocity minus the end-diastolic velocity divided by the maximal velocity. Increase in renal artery RI is seen in RAS, transplant rejection, acute tubular necrosis, graft infections, and obstructive hydronephrosis. The RI tends to be high in patients with chronic renal disease.
Goldblatt kidney is a condition where the kidney starts to release rennin to overcome RAS, leading to renovascular hypertension. Page kidney, on the other hand, is a condition where the kidney is compressed from an adjacent pathology that causes cortical ischemia. The kidney releases rennin to overcome the ischemia, leading to renovascular hypertension.
The Normal Renal Artery Waveform Parameters
The normal renal artery waveform shows low resistance, continuous profile through the cardiac cycle, with an RI <0.7. Also, the normal main renal artery waveform has an early systolic peak (ESP) (Fig. 4.1.3).

Fig. 4.1.3
Color Doppler sonogram of the renal arteries demonstrates normal arterial waveform of the right renal artery (a) and the left renal artery (b)
The renal-aortic ration (RAR) is defined as the maximum peak systolic velocity (PSV) of the renal artery divided by the maximum PSV of the aorta at the level of the superior mesenteric artery (SMA). A high false RAR can be seen in cases of abdominal aortic aneurysm, and aortic PSV <40 cm/s, or aortic PSV >125 cm/s. Also, RAR should not be used in the assessment of renal artery aneurysm for young patients or patients with renal artery stents.
The normal interlobar and segmental arteries display an ESP at the beginning of the systole. The ESP is absent when the arterial stenosis is >60 %. The Doppler angle should be <30°; otherwise, the peak will not be demonstrated.
The systolic acceleration time (SAT) is defined as the time measured from the start of the systolic upstroke to the first ESP. Normally it is <0.07.
Signs of Direct RAS on Doppler Sonography
High renal parenchymal echogenicity that may reach or exceed the liver echogenicity (signs of renal parenchymal damage). A kidney disease can cause renal artery-resistant waveform, which may be mistaken with RAS.
Normal RAR (<3.5) and the normal renal PSV (<180 cm/s). Sixty percent RAS shows RAR <3.5, with PSV between 180 and 200 cm/s. There is no poststenotic turbulence (mosaic pattern/aliasing artifact) with RAS <60 %. A 60–99 % RAS shows RAR >3.5, PSV >200 cm/s, and poststenotic turbulence.
Signs on Indirect RAS on Doppler Sonography
Absence of the ESP.
An accelerated time peak >100 ms is consistent with >60 % stenosis.
Tardus parvus waveform consists of slow, damped systolic acceleration (tardus) and rounding and flattening of the systolic peak (parvus).
More than (−5) difference between the two kidneys RI.
Kidney size <9 cm or the difference in size between the two kidneys >2 cm in diameter (normal kidney size = 9–12 cm in diameter).
Signs on CT
Arterial cut-off sign: the course of the renal artery is seen interrupted on contrast-enhanced images. It is a sign of renal artery obstruction (Fig. 4.1.4).
Rim sign of vascular compromise: the kidney fails to enhance on contrast-enhanced images, with a thin rim of subcapsular enhancement seen paralleling the renal margin (Fig. 4.1.4). This sign is caused by renal medullary arterial perfusion interruption in cases of renal artery obstruction, with preserved perfusion of the renal cortex by the capsular perforating vessels. This sign can be seen in cases of renal vein thrombosis, renal arterial occlusion, and acute tubular necrosis.
Reverse rim sign: this sign is seen as hypodense renal cortex against a contrast-enhanced background of intact medullary arterial perfusion (Fig. 4.1.4). This sign is seen in cases of compromised renal arterial perfusion in cases of cortical necrosis.

Fig. 4.1.4
Axial postcontrast-enhanced CT illustration shows the arterial cut-off sign (solid arrowhead), rim sign of vascular compromise (open arrowhead), and reverse rim sign (arrow)
Signs on MRA
Magnetic resonance angiography (MRA) demonstrates the aortic vessels clearly, and it is mostly used after detection of RAS on Doppler sonography for surgical planning (Fig. 4.1.5).

Fig. 4.1.5
MRA image shows >50 % stenosis of the left renal artery (arrowhead) in a patient with peripheral vascular disease
Coarctation of the Aorta
Coarctation of the aorta is a condition characterized by aortic lumen narrowing, most commonly located below the origin of the left subclavian artery (the aortic isthmus near the ligamentum arteriosum), causing hypertension below the level of the narrowing.
Patients with aortic coarctation often present with severe hypertension that does not respond to antihypertensive medication and chest pain that radiates to the back. Patients with aortic coarctation carry the risk of aortic dissection, which is characterized by aortic wall intimal tear and leaking of the blood in between the aortic wall layers.
When it is chronic, aortic coarctation causes dilatation of the internal mammary and intercostals arteries. Chronic high-pressure pulsation of the intercostals arteries over the inferior aspect of the ribs may result in rib notching. Coarctation of the aorta can be seen in up to 30 % in patients with bicuspid aortic valve.
Pseudocoarctation of the aorta is a relatively rare condition characterized by kinking of the aorta at its isthmus near the ligamentum arteriosum without lumen narrowing. Pseudocoarctation resembles true coarctation; differentiation is possible by looking for changes of the collateral circulation, where there will be no enlarged peripheral vessels, signs of pressure gradients, or rib notching. The condition is symptomless and can be associated with congenital bicuspid aortic valve.
Signs on Chest Radiograph
In chronic cases of aortic coarctation, the ribs show irregular lower border representing rib notching (Fig. 4.1.6). This condition can be also seen in other conditions affecting the intercostals neurovascular bundle like superior vena cava obstruction syndrome and neurofibromatosis of the intercostals nerves.
The aortic arch is often bulging with dilatation of the aortic knuckle (Fig. 4.1.7).
Pseudocoarctation of the aorta is seen as elongated aortic knuckle appearing like a posterior mediastinal mass.

Fig. 4.1.6
Plain chest radiograph shows rib notching in a patient with severe aortic coarctation since 12 years (arrowheads)

Fig. 4.1.7
Posteroanterior plain chest radiograph in a patient with coarctation of the aorta shows bulging of the aortic knuckle
Signs on CT and MRI
Aortic coarctation classically is seen in sagittal reformatted images as a narrowing of the aortic lumen located at the area of the aortic isthmus, giving the classic “inverted shape of 3” (Fig. 4.1.8).
Aortic dissection is seen as hypodense layer found within the aortic lumen on postcontrast-enhanced images representing the false lumen (Fig. 4.1.9). Stanford type A dissection involves dissection of the ascending aorta and the arch up to the origin of the left subclavian artery. Stanford type B dissection involves dissection of the descending aorta below the origin of the subclavian artery. Type A is managed surgically, while type B is managed medically, providing evidence of end-organ ischemia not being present.
Aortic pseudocoarctation is seen on axial CT images as higher than normal located arch, with a well-defined enhancing vascular mass located near the aortic arch, representing the kinked portion of the arch.

Fig. 4.1.8
Sagittal MRA of a patient with coarctation of the aorta shows the inverted shape of 3, which is a characteristic of this condition (arrowhead)

Fig. 4.1.9
Axial CT angiography shows the classical intimal flap in a patient with aortic dissection Stanford type A
Polyarteritis Nodosa
Polyarteritis nodosa is a rare disease characterized by aneurysmal, nodular lesions that affect the medium-sized and small-sized arteries due to fibrinoid necrotizing vasculitis.
Vasculitides are a diverse group of diseases characterized by inflammation and necrosis of all three coats of the vessels (intima, media, and adventitia). They are divided into large-sized vessel vasculitis (e.g., Takayasu and giant cell arteritis), medium-sized vessel vasculitis (e.g., polyarteritis nodosa and Kawasaki disease), and small-sized vessel vasculitis (e.g., Wegener’s granulomatosis and Henoch–Schönlein purpura).
Polyarteritis nodosa commonly causes multiple arterial aneurismal wall formation, plus fragmentation and degeneration of the adventitia layer. Necrosis starts in the media layer and spreads to involve the entire width of the vascular wall. This inflammation and necrosis are associated with eosinophilic infiltration, fibrin deposition, and destruction of the elastic tissue and the intima layer. Vascular thrombosis or an aneurysm, mainly at the vessels bifurcation or the hilar region of viscera, may occur. Later, a granuloma is formed at the area of previous inflammation with fibroblast proliferation. A healed stage is characterized by vascular recanalization and formation of a nonvascularized fibrous mass that completely replaces a section of the vessel wall. The disease rapidly progresses once started, and death may result from strokes or myocardial infarctions within 2–3 months after onset.
Any organ can be affected by PAN, including the central and peripheral nervous system. The kidney is the most commonly involved (80–90 %), followed by the gastrointestinal (GI) tract (50–70 %). Clinically, patients present with vague signs and symptoms like fever, myalgia, headache, and malaise. Renal artery involvement, especially at the renal hilum, often results in rapidly progressing hypertension. Involvement of the GI tract may present with lower GI bleeding, abdominal pain, and vomiting (6 % of cases). Peripheral nervous system involvement compromises the nervous tissue blood supply causing polyneuropathy. Other lesions involve pericarditis, myocardial infarction, and purpuric skin rash. Up to 30 % of patients test positive to hepatitis B surface antigens, and up to 50 % of patients have arthralgias.
Laboratory investigations usually show leukocytosis with eosinophilia (4 %), anemia, uremia, and high erythrocyte sedimentation rate.
Signs on Angiography or MRA
Polyarteritis nodosa is suggested by detection of multiple aneurysms up to 1 cm in diameter within the renal, mesenteric, hepatic, or central venous vasculature. This finding is not pathognomonic since it can be seen in patients with necrotizing angiitis associated with drug abuse. The history and the high suspicion of PAV with this angiographic picture help to differentiate the two entities.
Signs on CT
The bowel walls are thickened and show white-attenuated target sign after contrast injection.
CT angiography shows multiple aortic aneurysms in the branches of the SMA or the celiac trunk.
In spotted nephrogram, small vessel occlusion in cases of PAN may cause patchy perfusion of the kidney on IVU or contrast-enhanced CT due to multiple infarctions (Fig. 4.1.10). This sign can be also seen in scleroderma and hypertensive nephrocalcinosis.

Fig. 4.1.10
Axial CT illustration demonstrates the spotted nephrogram that may be seen in patients with polyarteritis nodosa
Takayasu Arteritis
Takayasu arteritis is a rare, granulomatous disease characterized by inflammation of the large vessel walls, leading to progressive stenosis, aneurysms, or limb ischemia. The disease affects mainly the aorta and its major branches.
TA affects mainly young females between 20 and 30 years of age, who present with nonspecific systemic inflammatory symptoms like fever, night sweat, weight loss, myalgia, and arthralgia. TA has two main phases with different clinical presentations, an early and a late phase. The early phase is characterized by nonspecific inflammation in the blood vessels walls affecting the media and the adventitia layers. These nonspecific inflammatory changes are responsible for the nonspecific, systemic inflammatory symptoms. In contrast, the late phase is characterized by arterial symptoms with no systemic symptoms. The absence of the systemic symptoms in the late phase is attributed to the development of systemic collaterals; however, this is not seen in all patients. In the late phase, the coronary arteries might be affected. Hypertension, arterial stenosis, aneurysms, and dissection all can be seen in late phase of TA.
Criteria for TA Diagnosis (TA Diagnosis Requires at Least Three Criteria)
Age of presentation <40 years.
Limbs claudication.
Decrease one or both brachial arteries pulse.
Blood pressure difference of the extremities >10 mmHg.
Bruit heard over the aorta or the subclavian arteries.
Angiographic abnormalities include arterial occlusion, stenosis, or aneurysms of the aorta or its main branches. Commonly, these abnormalities are observed bilaterally.
Signs on CT and CT Angiography
In the acute phase, there is thickening of the great vessel wall without signs of calcification due to inflammation and intramural hematomas (especially the aorta) (Fig. 4.1.11).
The thickened vascular wall may show enhancement in early contrast phase due to inflammatory hyperemia.
Aneurysmal dilatation of the aortic root or its major branches is mainly observed in late chronic phase of the disease.
Signs of dissection, stenosis, or occlusion of the major vessels may be seen (Fig. 4.1.12).