Neuroendocrine neoplasms are a heterogeneous group of tumors arising from cells distributed throughout the body. Local and regional disease is managed with surgical resection; however, treatment of higher-grade neuroendocrine tumors (NETs), unresectable or metastatic disease is complex involving a combination of systemic targeted agents, transarterial embolization, and peptide receptor targeted therapies and is discussed in detail. The most important concept in modern NET workup is that an optimal diagnostic strategy requires combination of both anatomic and functional imaging modalities. NETs often present with unknown primary site of disease, and 68 Ga-DOTATATE PET can now diagnose these lesions with great sensitivity.
Key points
- •
Accurate neuroendocrine tumor (NET) imaging assessment and follow-up require familiarity with the entire range of disease sites, morphologic manifestations, as well as the impact of the various therapeutic options on clinical course.
- •
The variable conspicuity of NETs requires multiple phases of contrast enhancement to detect both tumor recurrence and metastasis. Although these tumors are typically hypervascular, they may exhibit little or no enhancement on the arterial phase and be optimally identified only on the porto-venous phase.
- •
68 Ga-DOTA-SSTR imaging is indicated for initial staging, detection of occult disease, diagnosis of recurrence, patient selection before peptide receptor radionuclide therapy, and disease confirmation in sites not amenable to biopsy.
- •
Transcatheter arterial embolization strategies show similar response outcomes, but differ importantly on their toxicity with reports of increased risk for biliary injury in patients with NET after chemoembolization using drug-eluting beads (DEB-TACE).
- •
Objective measurements used in RECIST 1.1 do not always offer the most accurate evaluation for targeted therapy response. Alternative response criteria using lesion attenuation and morphology offer promising results and seem better suited to assess response to target agents.
Introduction
Neuroendocrine neoplasms are a heterogeneous group of tumors that arise from cells that are distributed throughout the body, including central nervous system, thyroid, parathyroid glands, larynx, breast, as well as the respiratory, gastrointestinal, and urogenital tracts. The lungs (22%–27%) and gastrointestinal tract (62%–67%) are the most common primary sites. Given the wide variety of disease sites and clinical behavior, the treatment of neuroendocrine tumors (NETs) requires a multidisciplinary approach. Endocrine surgeons offer curative treatment for patients with locoregional tumors, endocrinologists and gastrointestinal medical oncologists offer a gamut of systemic therapeutic options, whereas radiologists aid in the diagnosis, treatment, and palliation of unresectable metastatic disease.
NETs account for approximately 0.5% of all newly diagnosed malignancies. The incidence is approximately 6.98/100,000 per year. , These tumors can arise in association with multiple endocrine neoplasia syndrome, von Hippel-Lindau syndrome, neurofibromatosis, tuberous sclerosis, but are in the great majority sporadic in nature. The management of NETs is predicated on histologic classification of NETs, which separates tumors into well-differentiated (G1) characterized by slow growth; moderately differentiated (G2) with heterogeneous differentiation; (G3) displaying poor-differentiation; and undifferentiated or anaplastic (G4). Imaging plays a central role in the diagnosis, workup, and monitoring of patients with NET. Establishing the site of the primary tumor has critical prognostic implications, as a combination of NET site, grade, and stage (local, regional, or distant) are the determinants of management and clinical outcome. Patients with primary rectal NETs have the best prognosis, followed by small intestine, lung/bronchus, stomach, and colon. Patients with pancreatic NET have the highest mortality risk.
Local and regional disease is typically managed with surgical resection, whenever possible. However, the treatment of patients with NET with higher grade, unresectable or metastatic disease can be complex involving a combination of systemic targeted agents, as well as transarterial embolization and peptide receptor targeted therapies and will be discussed in detail. The most important concept in modern NET workup is that an optimal diagnostic strategy requires combination of both anatomic and functional imaging modalities. This combined approach enables a more accurate identification of the primary disease site and disease staging. Because of their unique symptomatology, NETs often present with unknown primary site of disease, despite adequate cross-sectional imaging workup, and 68 Ga-DOTATATE PET can now diagnose these lesions with great sensitivity. Finally, functional imaging is essential to select ideal candidates for peptide receptor radionuclide therapy (PRRT).
Treatment considerations and expected outcomes
In cases with limited metastases, surgical resection of the primary tumor and metastases is possible. Overall survival rate was 50.4% at 10 years after resection in one study, and systematic review estimates overall survival to be 41% to 100% at 5 years after resection. Surgical resection of gastrointestinal NETs showed overall survival rates to be 84%, 67%, and 31% at 5 years, 10 years, and 20 years after resection, respectively, with overall median survival at 161 months. Recurrence after resection is expected, but decreasing tumor burden has been shown to improve symptoms and quality of life in patients.
Imaging is an integral part of the management and follow-up of patients with advanced locoregional or distant disease. Approximately 40% to 50% of patients with NET present with metastatic disease at initial diagnosis. Treatment for these patients encompasses long established approaches, such as somatostatin analogs to reduce both tumor growth and symptomatology and liver-directed therapies, reserved for patients with metastatic liver disease, particularly those with poorly controlled carcinoid syndrome. Modern approaches using precision medicine targeted therapies are used as systemic antitumor agents, and finally, peptide receptor radionuclides therapies are typically indicated for metastatic tumors expressing somatostatin receptors. Familiarity with the expected objective response and time to progression of each of these therapeutic approaches is required to establish optimal treatment surveillance strategies.
Somatostatin analogs
Somatostatin analogs (SSAs), namely octreotide and lanreotide, were originally used to modulate hormonal effects and growth of NET tumor cells by binding somatostatin receptors. These agents remain as the first-line management and therapy for NETs and have a remarkably successful history over decades. These therapies take advantage of somatostatin receptor overexpression characteristic of NETs. Initially, these agents were used mainly to regulate symptoms of hormone excess from carcinoid syndrome; they are now increasingly used also to control tumor growth. Octreotide was shown to increase median times to tumor progression to 14.3 months as compared with 6 months in the placebo group (PROMID trial, P = .000072). Stable disease was maintained in 66.7% of the octreotide group and 37.2% of the placebo group after 6 months of treatment. Lanreotide treatment for 2 years was shown to increase progression-free survival (PFS) in patients with locally advanced or metastatic nonfunctioning pancreatic or intestinal NETs as compared with placebo (CLARINET study). Length of PFS was not obtained in the lanreotide group but was shown to be at least 24 months and is estimated to be 32.8 months during open label data collection.
Image-guided liver-directed therapies
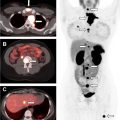
Advances in image-guided liver-directed therapies have led to improved outcomes for patients with liver metastases ( Fig. 1 ). Improvements in catheters, embolic agents, chemotherapy drugs, and delivery systems have been linked to further technical breakthroughs sparking interest in combination approaches with systemic therapies. Median overall survival for patients with NET with liver metastases is less than 5 years. Image-guided tumor ablation is considered a potential first-line treatment in many patients with single and/or small liver tumors, and it can be accomplished using chemical agents or thermal energy. Chemical ablation can be achieved by direct intratumoral percutaneous ethanol injection and, less commonly, ablation using acetic acid or chemotherapeutic agents that induce tumor cell death. Thermal ablation modalities include high-energy radiofrequency ablation (RFA) and microwave ablation (MWA); these procedures can be performed under imaging guidance by interventional radiologists or by surgeons in the operating suite. A systematic review of studies evaluating the use of ablative techniques for neuroendocrine liver metastases combining both percutaneous (26%) and surgical (74%) approaches for a total 595 patients showed local recurrence rates of approximately 20% on contrast-enhanced computed tomography (CT) of the thorax, abdomen, and pelvis within 2 years. Transarterial embolization, chemoembolization, and radioembolization consist of transcatheter intra-arterial delivery of a single or combination of embolic agents, chemotherapy drugs, or radioactive microspheres into a liver tumor. These treatments are based on the fact that blood flow to liver neoplasms is supplied virtually entirely through the hepatic artery, whereas supply to the normal liver parenchyma occurs predominantly through the portal vein. Moreover, tumor ischemia caused by embolization of the dominant arterial supply has a synergistic effect with the chemotherapeutic drugs. Only retrospective data are available comparing the different modalities in metastatic NET context with expected imaging response rates ranging between 60% to 95%. A retrospective multicenter study assessing 155 patients with NET with liver metastases undergoing conventional transarterial chemoembolization (TACE) (n = 50), transarterial radioembolization (TARE) (n = 64), and transarterial embolization (n = 41) demonstrated equivalency between the different modalities without significant differences. Radioembolization showed a higher hazard ratio for overall survival than chemoembolization (hazard ratio 1.8, P = .11). A more recent retrospective study of 248 patients from 2 academic medical institutions comparing TACE (79%) and TARE (21%) showed no difference in median overall survival, but disease control rate was greater for TACE on first posttreatment imaging. There was also no difference in PFS between TARE versus TACE (15.9 months vs 19.9 months). Despite their similar objective response outcomes, these different embolization strategies differ importantly on their toxicity with reports of increased risk for biliary injury in patients with NET after chemoembolization using drug-eluting beads transarterial chemoembolization (DEB-TACE). , Based on these studies, attention should be directed at diagnosing early bile duct dilatation and intrahepatic bilomas throughout DEB-TACE follow-up, since they may require percutaneous drainage.

New targeted agents
Better understanding of the molecular underpinnings of NET tumor growth has led to increased role for molecular targeted agents in the treatment of these tumors. Everolimus is an mTOR inhibitor that is, used for patients with progressive, metastatic gastrointestinal or bronchopulmonary tumors. In the RADIANT-4 study, 302 patients with progressive nonfunctional gastrointestinal tract or lung NET were randomized to receive either everolimus or placebo. Median PFS for the study was 11 months for patients undergoing treatment with everolimus versus 3.9 months with placebo. Everolimus was associated with a 52% reduction in the estimated risk of progression or death (hazard ratio 0.48, P <.00001) with infrequent grade 3 or 4 adverse events. National Comprehensive Cancer Network (NCCN) NET guidelines recommend everolimus for patients with progressive metastatic gastrointestinal tract NETs. In addition, a recent subgroup analysis of the RADIANT-4 lung patients showed a median PFS improvement of 5.6 months with similar safety profile supporting the use of the agent in patients with advanced nonfunctional lung NET, as well. Vascular endothelial growth factor (VEGF), a key driver of angiogenesis, is also implicated in NET progression with prognostic implications. In addition, NET tissues also exhibit overexpression of platelet-derived growth factor receptors (PDGFRs) and stem-cell factor receptor (c-kit). Sunitinib is a tyrosine kinase inhibitor that exerts its antitumor activity by inhibiting several of these pathways. A randomized, double-blind, placebo-controlled phase 3 trial of sunitinib in patients with advanced, well-differentiated pancreatic neuroendocrine tumors showed improved PFS (11.4 months in treatment group vs 5.5 months in placebo group) and objective response rate of 9.3% in the sunitinib group.
Peptide receptor radionuclide therapy
In the past decade, 68 Ga-labeled SSTR analog PET/CT has emerged as a more sensitive imaging solution for NETs, due to the increased affinity of these agents for somatostatin receptors subtype 2. , Thus, the next logical therapeutic step consisted of combining of one of these imaging isotopes with a therapeutic radioactive element, to develop a targeted theranostic agent. PRRT is a synthetic somatostatin analog that is radiolabeled with lutetium 177 ( 177 Lu-DOTATATE). , PRRT is emerging as the standard of care for patients with inoperable low-grade (1 or 2) NETs expressing somatostatin receptors. Once bound to the SSTR 2 receptors, the radionuclide is internalized, and cell death ensues due to lethal beta radiation. The 2 most common radionuclides used for PRRT are [yttrium-90 DOTA Phe-1-Tyr 3 -] Octreotide ( 90 Y-DOTATOC) and [Lutetitium-177 DOTA-phe1-Tyr 3 ]Octreotate ( 177 Lu-DOTATATE). In 2018, 177 Lu-DOTATATE (Lutathera; Advanced Accelerator Applications, Milburn, NJ) was approved by the US Food and Drug Administration for the treatment of SSTR-positive gastroenteropancreatic NETs, including foregut, midgut, and hindgut tumors. NCCN guidelines recommend Lu177-DOTATATE for low-grade and intermediate-grade patients with NET who progress on SSAs. A phase III randomized study (NETTER-1) evaluated 229 patients assigned to 177 Lu -DOTATATE or high-dose octreotide. Patients treated with PRRT showed significant improvement in PFS (not reached vs 8.4 months) with objective imaging response observed in 18% of patients. A subsequent study including 610 patients with metastatic gastroenteropancreatic and bronchial NETs showed PFS and overall survival for all patients of 29 months and 63 months.
Imaging treatment response and follow-up
Optimal NET imaging assessment and follow-up require familiarity the entire range of disease sites, morphologic manifestations, as well as the impact of the various therapeutic options on their clinical course. Meticulous evaluation requires both subjective and objective assessments, the latter fundamental in the context of clinical trials and currently dominated by size measurement(s). Changes in lesion attenuation on CT, MR imaging, and PET/CT can also be used as objective criteria of response, but these criteria are not as universally accepted as changes in tumor size defined by RECIST criteria. An accurate assessment of treatment response should rely on changes in lesion size and number; presence and magnitude of contrast enhancement; and finally changes in functional imaging. In addition to cursory measurements of primary and nodal sites, an active search for small peritoneal implants and bone metastases should be systematically carried out when evaluating follow-up scans for neuroendocrine patients. The indolent nature of NETs often translates into several consecutive imaging studies with minimal deviation from baseline scans. Typically, imaging surveillance requires more than a year to allow recognition of any appreciable change. Finally, because most well-differentiated NETs display increased vascular density, a salient feature of NETs is their exuberant enhancement on contrast-enhanced studies. Thus, it is important to recognize that, when subjected to antivascular therapies, objective response rates may not provide the full picture of treatment response; incorporation of both size and magnitude of lesion enhancement is needed to more accurately predict drug efficacy.
Anatomic imaging
Multi-detector CT (MDCT) is a widely available technique, with a high spatial and temporal resolution as well as multi-planar capabilities, which makes it one of the modalities of choice in the anatomic assessment of NETs. NETs are generally imaged using a multiphase imaging protocol that incorporates precontrast scans followed by late arterial phase (25–30 seconds), porto-venous phase and delayed imaging. This technique should be obtained with 100 to 120 mL of iodinated contrast (Omnipaque 350; GE Health Care, Princeton, NJ) injected at 3 to 5 mL/s with slices reconstructed at 2.5 to 5 mm. The requirement of multiple phases of contrast enhancement reflects the variable conspicuity of these tumors, seen in both the primary tumor and liver metastases. In some studies, the tumors are more conspicuous on the arterial phase, and in other studies they are better seen on the porto-venous phase. This is because although these tumors are traditionally viewed as hypervascular, they may have little or no enhancement on the arterial phase and may be optimally identified only on the portal phase. The precontrast series is an underappreciated but essential element of the imaging protocol for NET liver metastasis, as some liver metastases may only be evident on this acquisition. This basic multiphase CT protocol is not only ideal for the diagnosis and follow-up liver metastases but also for the detection and definition of regional extent of pancreatic neuro-endocrine tumors. However, the protocol needs slight modification for the localization of small bowel tumors; this evaluation may be performed with CT enterography using a negative or low attenuating oral contrast agent to distend the bowel.
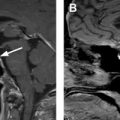
Regarding liver metastases, MR imaging provides an alternative and reliable imaging modality for NET surveillance ( Fig. 2 ). The imaging protocol relies on a similar use of multiphase postcontrast imaging to CT, using a T1-weighted fat-suppressed breath-hold 3-dimensional gradient-recalled echo sequence and includes the acquisition of precontrast, arterial phase, porto-venous, equilibrium and delayed phases, (typical parameters 3 mm, 288 × 155, 1 NEX). In addition, the protocol includes a T2-weighted fast spin echo sequence with fat suppression (5 mm, 128 × 156, 3 NEX), a diffusion-weighted sequence (b values 0, 600, 1000; 5 mm, 128 × 102, 6 NEX) ( Table 1 ). The use of diffusion-weighted imaging has shown improved sensitivity in the detection and characterization of NET liver metastasis. When needed for problem solving, MR imaging can be performed with liver-specific contrast, where the absence of contrast accumulation within the tumor on the images acquired 20 minutes after injection allows for a sharp tumor-to-normal liver interface. One downside to keep in mind when using exclusively MR imaging for follow-up is the greater difficulty to detect small peritoneal implants in comparison to CT.