Head and neck high-flow vascular malformations are uncommon lesions whose management presents a clinical challenge. Although in some rare cases a complete cure is possible, in the vast majority the primary objective is symptom control, cosmesis improvement, and preservation of vital functions. Striving for “complete” treatment in most cases results in potentially devastating clinical and cosmetic outcome. Collateral supply via intracranial vessels is not uncommon, and scrupulous efforts to avoid complications related to inadvertent intracranial embolization or venous thrombosis are mandatory. Regardless of therapeutic goal, close long-term follow-up for lesion recurrence is necessary. Recent demonstration of syndromic associations for some subsets of HFVMs holds out the promise of the future development of medical therapy for these difficult lesions.
Vascular malformations of the head and neck are rare lesions, thought to result from errors in vascular morphogenesis. These lesions can be subdivided either histologically based on the predominant vascular channel type, or functionally based on the flow characteristics, ie, high-flow versus low-flow lesions.
Our focus is on the high-flow vascular malformations (HFVMs) of the head and neck, largely arteriovenous malformations (AVM), and arteriovenous fistulae (AVF). Other vascular lesions with high-flow characteristics, such as infantile hemangiomas during their proliferative stage and other vascular tumors, are outside our purview; these other lesions have natural histories and treatment paradigms that fundamentally differ from the vascular malformations we consider here.
The high-flow lesions have arteriovenous shunting as an intrinsic feature, ie, shunting of blood under arterial pressure and arterial flow rates into the venous system; herein is the root of much of the pathophysiology of these lesions. The AV shunt presents a risk of hemorrhage, most commonly from rupture of venous structures not designed for arterial pressure, although arterial rupture, particularly at weak points such as flow-related or intranidal aneurysms, certainly occurs as well. Additionally, the AV shunt likely causes a localized steal phenomenon, with chronically ischemic tissue in the vicinity of the AVM leading to pain, infection, skin and mucosal breakdown, and so forth.
The morphology of the shunt can take two forms: (1) a fistula, or a direct communication from an artery of visible caliber into a vein of visible caliber, or (2) a nidus, a network of abnormal vascular channels bridging the feeding arteries and draining veins. In either case, normal arterioles and the capillary bed are absent. AVMs and fistulae are not mutually exclusive, as it is not uncommon to see fistulous foci within a complex AVM nidus. Compared with the low-flow vascular lesions of the head and neck reviewed elsewhere in this issue, the HFVMs are rare. The rarity of these lesions and the complexity of the pathophysiology pose a significant management challenge, and no standard treatment paradigm has been established.
Proposed treatment algorithms, however, usually involve sclerotherapy, endovascular or percutaneous embolization, surgical excision, or some combination of these modalities. In this article, we focus on the endovascular treatments for various HFVMs (mainly AVMs) of the head and neck, as proposed in the literature, and elaborate on our approach to these lesions. By way of definition, by “sclerotherapy” we refer to the injection of an agent designed to ultimately convert a vascular space into a scar, typically by inducing a cascade of inflammation and thrombosis. Sclerosants are almost always injected percutaneously or transmucosally (as described elsewhere in this issue), although transarterial injection of absolute ethanol would be an example of intravascular sclerotherapy. By “embolization,” we refer to the injection or deployment of a largely inert substance designed to impede flow or passively fill a vascular space. Examples of liquid embolics include n-butyl cyanoacrylate (n-BCA) and Onyx, whereas examples of solid embolics include polyvinyl alcohol particles (PVA) and coils.
Incidence and clinical presentation
The pathogenesis of vascular malformations is complex and incompletely understood. Postulated mechanisms have been summarized in several recent reviews. Rather than representing neoplasms, these lesions result from errors in vascular development.
HFVMs of the head and neck are quite rare and their true incidence is unknown. There may be a slightly higher prevalence in girls, with at least one series reporting a female-to-male ratio of 1.5:1. The lesions are likely present at birth, but many are not clinically evident until later in life. Although clinical presentation is most common between late infancy and early school age, the potential age of presentation varies from the neonatal period to middle age. HFVMs usually enlarge in proportion with the growth of the child, but rapid progression can be provoked by hormonal changes (eg, puberty, pregnancy) or trauma (eg, direct force, surgical intervention, infection, thrombosis). The associated signs and symptoms are largely dependent upon the extent of the lesion and the site of involvement in the head and neck.
The most common site for AVMs in the head and neck is the cheek (31%), followed by the ear (16%) and the nose (11%), whereas areas such as the mandible (5%) and maxilla (4%) are less commonly involved. Traditionally, the Schobinger staging system has been used to assess AVMs. In stage I, the lesions are quiescent and asymptomatic. In stage II, the lesions demonstrate expansion but remain largely asymptomatic. Overlying skin changes, increased local temperature, or palpable pulse and thrill are often present. In stage III, the lesions become symptomatic, most commonly with pain, ulceration, and hemorrhage. In stage IV, a high degree of AV shunting leads to cardiac decompensation. Despite its widespread use, it is debatable how valuable the Schobinger classification is in the head and neck—extracranial lesions are virtually never large enough to cause cardiac overload, the natural history is most often unpredictable rather than progressing neatly from stage to stage, and the decision to treat or not at a given point is not a strict function of the Schobinger stage. A treatment dilemma often occurs with the stage I lesions, which may remain stable for a long period of time. At this juncture, there is no convincing evidence that intervening early alters the natural history of HFVMs, and our practice is to typically closely follow stage I lesions expectantly, and delay treatment attempts until rapid progression (stage II) or complications (stage III) become evident. However, faced with a giant, difficult-to-manage AVM in a teenager or young adult, one cannot help but wonder retrospectively whether earlier intervention would have led to different clinical evolution.
Diagnosis
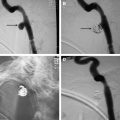
The diagnosis of a high-flow vascular malformation can be reliably made based on clinical history and physical exam. On exam, the high-flow lesions are usually reddish, warm, firm, and pulsatile ( Fig. 1 ). Local skin ischemia, ulceration, and/or hemorrhage are frequently seen with more advanced lesions ( Fig. 2 ). As described elsewhere in this issue, these are not typical features for low-flow lesions, and as such, the diagnosis is usually not dependent upon imaging results.
Cross-sectional imaging, including computerized tomographic angiography and magnetic resonance angiography, can be valuable in delineating the tissue spaces involved by the AVM and its effect on adjacent structures. However, catheter angiography continues to provide the highest spatial resolution available, as well as critical insight into the dynamics of flow in the lesion. The selection of images in this article reflects our bias in this regard.
For noninvasive cross-sectional imaging workup of HFVMs, magnetic resonance imaging (MR imaging) is the modality of choice because of its superior soft tissue contrast and ability to display the extent of the lesion. On MR imaging, HFVMs characteristically show dilated feeding arteries and draining veins with flow voids on T2-weighted imaging and corresponding hyperintense signal on flow-enhanced gradient-echo sequences. The nidus of an AVM may be delineated as smaller caliber curvilinear abnormalities, although often the extent of the nidus visualized by catheter angiography is significantly underestimated on MR. Newer sequences, such as time-resolved MR angiography and MR perfusion imaging, may further characterize the flow characteristics and hemodynamics. Computed tomography (CT) is crucial in delineating skeletal involvement, in particular the mandible, skull base, orbits, and calvarium. Color Doppler ultrasonography, portable and widely available, is helpful in verifying high-flow vascularity if the initial diagnosis is in doubt, and can accurately delineate the extent of the lesion, although this mainly holds for small superficial cases.
Diagnosis
The diagnosis of a high-flow vascular malformation can be reliably made based on clinical history and physical exam. On exam, the high-flow lesions are usually reddish, warm, firm, and pulsatile ( Fig. 1 ). Local skin ischemia, ulceration, and/or hemorrhage are frequently seen with more advanced lesions ( Fig. 2 ). As described elsewhere in this issue, these are not typical features for low-flow lesions, and as such, the diagnosis is usually not dependent upon imaging results.
Cross-sectional imaging, including computerized tomographic angiography and magnetic resonance angiography, can be valuable in delineating the tissue spaces involved by the AVM and its effect on adjacent structures. However, catheter angiography continues to provide the highest spatial resolution available, as well as critical insight into the dynamics of flow in the lesion. The selection of images in this article reflects our bias in this regard.
For noninvasive cross-sectional imaging workup of HFVMs, magnetic resonance imaging (MR imaging) is the modality of choice because of its superior soft tissue contrast and ability to display the extent of the lesion. On MR imaging, HFVMs characteristically show dilated feeding arteries and draining veins with flow voids on T2-weighted imaging and corresponding hyperintense signal on flow-enhanced gradient-echo sequences. The nidus of an AVM may be delineated as smaller caliber curvilinear abnormalities, although often the extent of the nidus visualized by catheter angiography is significantly underestimated on MR. Newer sequences, such as time-resolved MR angiography and MR perfusion imaging, may further characterize the flow characteristics and hemodynamics. Computed tomography (CT) is crucial in delineating skeletal involvement, in particular the mandible, skull base, orbits, and calvarium. Color Doppler ultrasonography, portable and widely available, is helpful in verifying high-flow vascularity if the initial diagnosis is in doubt, and can accurately delineate the extent of the lesion, although this mainly holds for small superficial cases.
Therapeutic goal and endovascular management
Although lethal complications such as massive hemorrhage do occur, HFVMs of the head and neck are usually not life-threatening. Given the inherently trans-spatial nature of these lesions, the therapeutic goal for most patients with complex lesions is symptomatic control, preservation of vital functions (eg, vision, hearing, or mastication), or aesthetic restoration, rather than a complete “cure.” Treatment options may depend on the site, size, and complexity of the lesion, as well as the experience and preference of the treating physicians. The available treatment modalities include percutaneous or endovascular embolization or sclerotherapy and surgical resection. A multidisciplinary approach is essential.
Some authors have advocated sclerotherapy as the initial treatment modality when the AVM nidus is accessible percutaneously, reserving the more invasive and potentially more disfiguring combined embolization/surgery for lesions resistant to sclerotherapy or inaccessible percutaneously. There are also reports of successful cure with embolization alone, but these seemed to be limited to smaller AVMs and long-term follow-up is lacking. The best chance for a complete cure of AVMs of the head and neck seems to be via a combination of preoperative embolization and surgical resection. If a complete resection is not achievable because of the extent of the lesion or involvement of vital structures, partial targeted endovascular embolization with liquid embolic agents is the treatment of choice.
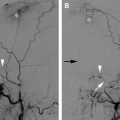
It is important to emphasize that, regardless of the intended outcomes, proximal embolization of feeding arteries is contraindicated, as is proximal surgical arterial ligation. These not only promote recruitment of nearby arteries to perfuse the nidus (see Fig. 2 ), but also limit future endovascular access to the nidus. By the same token, partial surgical resection of the AVM itself frequently (and perhaps inevitably) leads to progression, and therefore should be reserved only for very rare cases of life-threatening hemorrhage uncontrollable with endovascular or percutaneous embolization.
The ultimate target for endovascular embolization is occlusion of the nidus and initial segment of the venous outflow. Embolization may be curative, palliative (for symptomatic control of unresectable lesions), or preoperative, with the choice of agent depending on the intended outcome. For preoperative embolization, temporary occlusive agents such as gelfoam powder, PVA particles, and embospheres can be used. These agents very effectively reduce vascular inflow to AVMs, allowing for significantly lower rates of intraoperative blood loss. PVA and embospheres are available with different particulate diameters, allowing for initial targeting of distal arteriolar branches with small sizes, followed by occlusion of larger, more proximal branches with larger sizes. However, these agents are removed within weeks by phagocytosis, resulting in short-term revascularization. Thus, for embolization that is not to be followed by resection, permanent liquid agents capable of permeating the AVM nidus, such as absolute ethanol, n-BCA, or the more recently available Onyx, may be used.
Endovascular embolization with Onyx
The use of ethylene-vinyl alcohol copolymer (Onyx, EV3 Neurovascular, Irvine, CA) as an embolic agent for AVMs was first reported in the early 1990s in Japan. It has since become commercially available as a nonadhesive liquid embolic agent under the trade name of Onyx, and is approved by the Food and Drug Administration for use in embolizing brain AVMs.
The availability of Onyx has brought about a sea-change in the embolization of central nervous system AVMs. In contact with blood, Onyx precipitates on the surface while maintaining a liquid core, allowing the formation of a nonadhesive lava-like continuous mass. This lowers the risk of fragmentation leading to unintended distal embolization, and offers the user significantly more control with which to aggressively embolize even very extensive AVM nidus and multiple nidal compartments from a single catheter position; this was not feasible with other liquid agents. Its ability to maintain a constant liquid core allows for longer injections and better assessment of the treatment progress. In contrast to agents such as n-BCA or alcohol, Onyx elicits less inflammatory response and causes less endothelial damage and angionecrosis. Vessels embolized with Onyx are filled with a soft, sponge-like substance and are less fragile than vessels embolized with other agents, perhaps because of less of an inflammatory reaction; these factors likely contribute to its improved handling characteristics during surgical resection.
Despite these advantages in the central nervous system, reports on the use of Onyx for embolizing extracranial AVMs remain scarce. Onyx is black, and concerns have been raised regarding its potential for causing black skin staining for superficial lesions. However, we have found Onyx to be superficially invisible in almost all cases. The potential for skin staining seems to be limited to cases where the AVM has components that are superficial enough to have infiltrated dermal layers. This scenario will inevitably have already led to skin staining and darkening by the AVM itself, rendering any deposited Onyx inconspicuous. At our institution, Onyx has become the embolic agent of choice for treatment of AVMs in the head and neck, allowing us to embolize even the most complex lesions aggressively ( Figs. 3 and 4 ). Our experience with its use, both for preoperative and primary embolization, has been very positive.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree