Mulliken and Glowacki’s seminal classification of vascular anomalies into vascular tumors (with infantile hemangiomas being paradigmatic) versus nontumorous vascular malformations has been as important in the head and neck region as elsewhere. These latter are congenital, have an equal gender incidence, virtually always grow in size with the patient during childhood, and virtually never involute spontaneously. The vascular malformations can in turn be subclassified into high-flow and low-flow. Our focus is on the low-flow malformations, which include those with venous, lymphatic, and, to a lesser extent, capillary components. We address diagnostic and clinical characteristics, particularly insofar as they relate to the structures of the head and neck, and discuss neurointerventional management in some detail.
Mulliken and Glowacki’s seminal classification of vascular anomalies into vascular tumors (with infantile hemangiomas being paradigmatic) versus nontumorous vascular malformations has been as important in the head and neck region as elsewhere. These latter are congenital, have an equal gender incidence, virtually always grow in size with the patient during childhood, and virtually never involute spontaneously. The vascular malformations can in turn be subclassified into high-flow (ie, those with arterial components, such as arteriovenous malformations and fistulae) and low-flow (ie, those with no arterial components). Our focus here is entirely on the latter, the low-flow malformations, which include those with venous, lymphatic, and, to a lesser extent, capillary components. We address diagnostic and clinical characteristics, particularly insofar as they relate to the structures of the head and neck, and discuss neurointerventional management in some detail.
Clinicopathologic features
Capillary Malformations
Capillary malformations or stains, of which the facial port wine stain is the best known example, are flat, well demarcated, lesions, pink in infancy and tending to darken to purple with age ( Fig. 1 A ). These are to be differentiated from the very common fading macular stains (the “stork bite” and “angel kiss” being the most well known), which typically lighten or disappear. Histopathology demonstrates ectatic blood vessels in the dermis associated with reduction in innervation, which may play a role in the pathogenesis. Overall, there is a reported incidence of 0.3% at birth, with no known gender or ethnic predilections. They may be single or multiple, and have a predilection for the head and neck region.
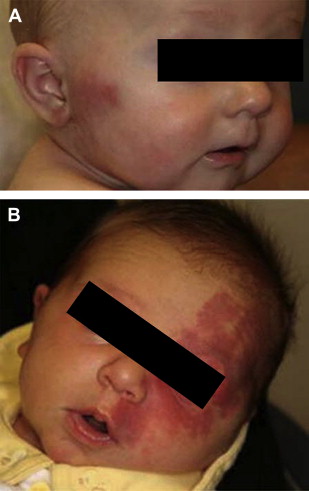
Capillary stains may occur in a dermatomal configuration, most commonly corresponding to the V1 and V2 branches of the trigeminal nerve ( Fig. 1 B). Whereas in most cases, the trigeminal dermatomal distribution occurs in isolation, in 3% to 8% of patients, there is an association with Sturge Weber syndrome and unilateral glaucoma. In the vast majority of cases with neuro-ocular involvement, the capillary malformation occurs in the V1 distribution, with or without involvement of V2 and V3.
Several syndromes include capillary stains within their list of clinical manifestations, the most familiar being Sturge-Weber syndrome; other examples (not necessarily involving head and neck capillary stains) are Klippel-Trenaunay syndrome, Parkes Weber syndrome, macrocephaly-capillary malformation syndrome, and capillary malformation-arteriovenous malformation syndrome (CM-AVM). CM-AVM has been recently linked to chromosome 5q14-21, with a defect in the RASA1 gene, which encodes a p120 Ras GTPase-activating protein, involved in cell adhesion and angiogenesis. Capillary malformations can be associated with overgrowth of the affected limb and even with hemihypertrophy. Klippel-Trenaunay syndrome consists of limb overgrowth with capillary malformation, venous anomalies, such as phlebectasia and hypoplasia, and lymphatic malformations.
In the vast majority of cases, capillary stains are only of clinical import because of issues of cosmesis and because of their syndromic association, although they have been reported to very rarely bleed profusely after minor trauma. Given the superficial nature of the lesions, imaging is typically done to exclude associated deeper lesions and associated neuro-ocular abnormalities, rather than to assess the capillary stain itself. Doppler ultrasound may be useful in cases where a deeper arteriovenous malformation is suspected. The current standard treatment is pulsed-dye laser, although only 15% to 20% of lesions clear completely. This technique has been reviewed elsewhere.
Venous Malformations
Venous malformations (VM) are congenital anomalies characterized by irregular endothelial-lined channels, with thin walls deficient in smooth muscle. When superficial enough to be appreciated externally, they typically have a bluish purplish hue, and are soft and compressible ( Fig. 2 A, B ). Head and neck venous malformations often expand when the patient is head-down, during Valsalva, and during other maneuvers that reduce venous return. Episodic focal thrombosis is nearly ubiquitous and may be associated with swelling and pain. Permanent phleboliths resulting from such episodes are common. The overall incidence of venous malformations is approximately 1 per 10,000. They are equally likely to occur in the head and neck region and in the extremities, with 40% found in each, with the remaining 20% seen in the trunk. As with other vascular malformations, swelling and enlargement can occur following trauma or hormonal changes, such as menarche or pregnancy.
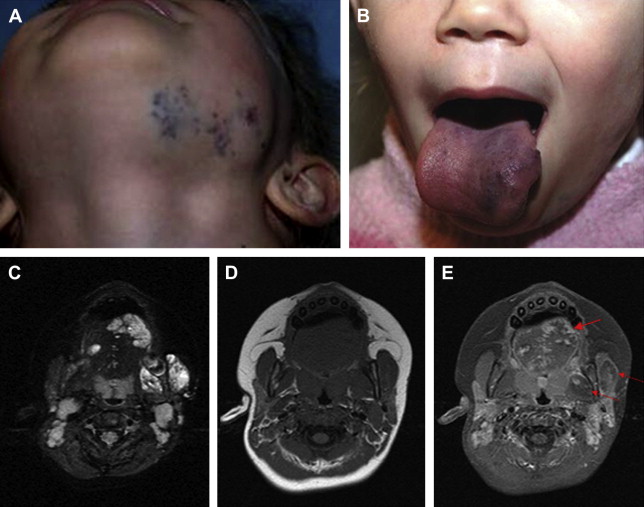
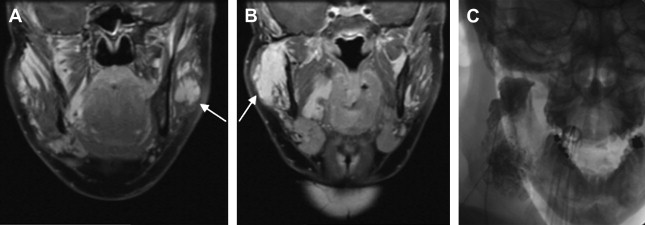
Pain associated with venous malformations is complex and multifactorial. Other than the above-mentioned thrombosis, intra-articular hemorrhage, muscle fibrosis, and bone and muscle deformity resulting in premature arthropathy can all serve as etiologies for pain and discomfort. In the head and neck in particular, the extent of the lesion is often greater than appreciated on clinical examination. Facial venous malformations frequently demonstrate extension into the deeper musculature and oral mucosa, and can present with oral bleeding ( Fig. 2 C–E). Similarly, there is often extension of temporal venous malformations into the parotid gland, and of neck venous malformations into the larynx or trepezius. Additional clinical manifestations typically relate to mass effect from a growing lesion within or adjacent to an important anatomic space, such as the orbit or airway ( Fig. 3 A, Fig. 2 B). Cosmesis is often an issue as well, with facial asymmetry inducing patients or their parents to seek treatment.
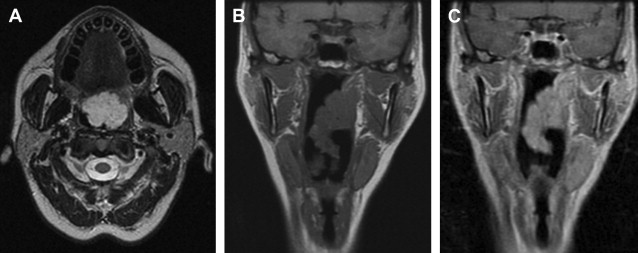
Venous malformations are associated with several syndromes, including glomuvenous malformation (glomulin mutation), cutaneomucosal venous malformation (TIE2 mutation), and blue rubber bleb nevus syndrome. Blue rubber bleb is a sporadic disorder with a plethora of venous malformations in the head and neck ( Fig. 4 ) as well as in the extremities ( Fig. 5 ), with the development of new lesions throughout life.
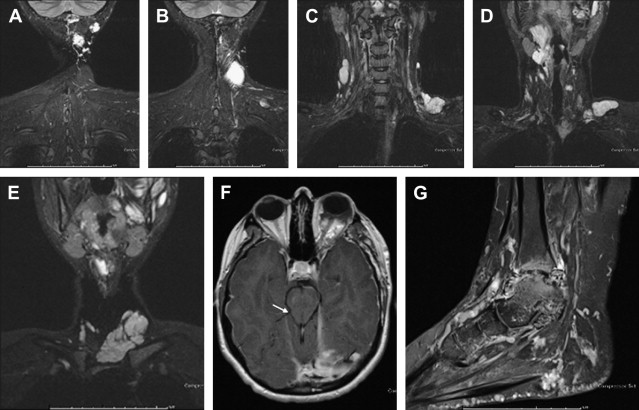
As manifestations of dysplastic veins, venous malformations by definition drain into the regional normal venous system. Given the typically large caliber of the anomalous venous sac relative to its low inflow, as well as the typically diminutive connectors between the malformation and adjacent normal veins, venous drainage is often delayed; rapid drainage can, however, occur. As we shall see, the rapidity, type, and particular pathway of venous drainage impacts on management strategy.
Lymphatic Malformations
Lymphatic malformations (LM) consist of cysts that are classified as either macrocystic, microcystic, or combined. Although strict numerical criteria for macrocysts have been proposed, we prefer to define as macrocystic any radiologically discernible cyst. As will be described later in this article, both the imaging characteristics and the response to treatment hinge crucially upon this distinction. Lymphatic malformations are particularly trans-spatial, crossing tissue planes and regional boundaries. The overlying skin or mucosal surfaces may demonstrate lymphatic vesicles (in microcystic cases).
Macrocystic lymphatic malformations have a decided predilection for the head and neck region, manifesting there 70% to 80% of the time; other locations include axillae in 20%, superior mediastinum, mesentery, retroperitoneum, pelvis, and lower limbs. Although they are congenital lesions, lymphatic malformations may not present immediately after birth ( Fig. 6 ). Clinical manifestations typically appear before the second year of life, however, owing to increasing mass effect. Sudden enlargement may follow infection or intralesional hemorrhage. Spontaneous involution, although unusual, has been reported. Numerous syndromes have been reported in association with LM, including Klippel-Trenaunay, Turner, Noonan, and trisomies 13 and 18. The incidence of lymphatic malformations is approximately 2.8 per 100,000 hospital admissions, but there are no accepted figures for overall population incidence.
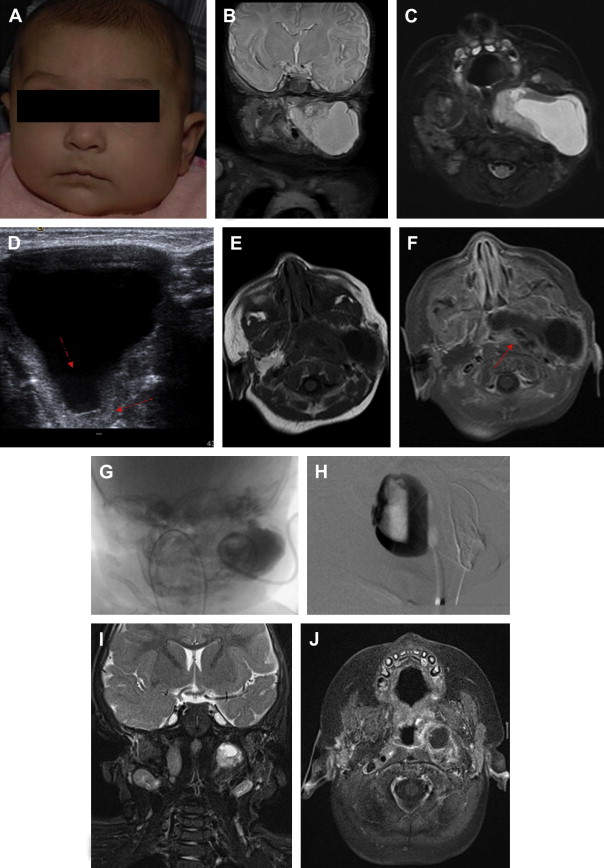
Morbidity associated with lymphatic malformations in the head and neck is primarily through recurrent infection, tissue overgrowth, mass effect on functionally important structures, skeletal hypertrophy, and via effects on cosmesis. In rare cases outside the head and neck, morbidity can be related to fluid depletion from massive chylous collections in the thorax or abdomen, or to functional impairment from diffuse lower extremity involvement.
Combined and Syndromic Low-Flow Malformations
Although frequently diagnosed in clinical imaging reports, the true incidence of combined or syndromic vascular anomalies, while unknown, is almost certainly very low. The frequent misdiagnosis results from atypical clinical and imaging appearance of a lesion (eg, a microcystic LM mimicking a VM, although not manifesting the classic imaging findings). Klippel-Trenaunay patients have all three major types of malformation present in the affected limb; hence the name capillary-lymphatic-venous malformations (CLVM).
Diagnosis: imaging characteristics
Low-flow vascular malformations can often be correctly diagnosed based on physical examination and history. Anomalous draining veins of venous malformations can on occasion be seen superficially, and VMs tend to be compressible, have a characteristic bluish color, and to expand when venous drainage is slowed. Lymphatic malformations are soft, noncompressible translucent masses with overlying normal or bluish skin, often with superficial vesicles (in microcystic cases). Nevertheless, imaging plays an essential role in characterizing these lesions to confirm the diagnosis (such as excluding the diagnosis of neoplasm), to define the spatial extent of the lesion and its relationship to important nearby structures, and for treatment planning.
Superficial and small lesions are well examined by ultrasound, with gray scale studies defining the extent and compressibility of the lesion, and spectral and color Doppler interrogation used to identify the flow characteristics. Sonography can also play a crucial role in differentiating macrocystic and microcystic lymphatic malformation ( Fig. 6 D). Very superficial lesions may be remarkably inconspicuous on MR imaging and yet well defined on ultrasound. Additionally, ultrasound has significant utility in providing image guidance for sclerotherapy, as described below.
However, in most cases, we have found MR imaging to be virtually indispensible. Its unparalleled contrast resolution more reliably determines the full extent of the lesion than any other modality. We most heavily rely on pre- and post-contrast T1-weighted images as well as T2-weighted images, and consider fat suppression to be critical in all MR sequences in the workup of vascular anomalies (see Fig. 6 ). Other MR techniques, such as MR angiography, venography, and lymphangiography, tend to contribute little in cases of low-flow anomalies. CT plays more of an adjunctive role in the workup of vascular malformations, being of most use in cases where bony involvement is crucial to delineate (as in sinus, skull base, cranium, or mandibular cases).
Untreated, both venous and lymphatic malformations typically appear hyperintense on T2 imaging, and the scope of the abnormal T2 signal can be used to define the overall extent of the lesion. After treatment by sclerotherapy, with the resultant progressive conversion of lesion to scar tissue, the T2 signal characteristically becomes less hyperintense. The enhancement pattern after contrast administration is a crucial differentiating feature, with venous malformations most characteristically enhancing avidly but in a patchy, heterogeneous pattern (see Figs. 2 to 4 ). In contrast, enhancement of lymphatic malformations is variable (see Fig. 6 ). Mild rim enhancement may be seen with macrocystic lymphatic malformations, with only minimal enhancement identified in microcystic LMs ( Fig. 7 ); however, in the setting of superinfection or inflammation, LMs may enhance avidly as well.
Venous malformations often display irregular intervening venous walls within the hyperintense areas, and occasionally adjacent enhancing serpentine vascular channels can be seen. Thrombi can have variable appearances, based on their age. Phleboliths are hypointense on all sequences on MR, are dense on CT, and are typically mobile (although confined to the venous space). They are hyperechoic and show acoustic shadowing on ultrasound. Although phleboliths have been thought to be virtually pathognomonic of venous malformations, they can rarely occur in lymphatic malformations as well. Venous malformations typically show compressible, confluent anechoic-hypoechoic channels on ultrasound (which are demonstrably venous on color Doppler imaging), separated by more solid regions of variable echogenicity ( Fig. 8 ).
The sonographic appearance of lymphatic malformations is dependent upon the type. Macrocystic lymphatic malformations show anechoic cysts, often containing internal debris or fluid-fluid levels resulting from episodes of hemorrhage. Internal septations are common and best visualized with sonography ( Fig. 9 ). Microcystic lymphatic malformations are ill-defined echoge-nic masses, showing diffuse involvement of surrounding tissue. On MR, macrocysts are T2 hyperintense and T1 hypointense, whereas on CT they are hypodense. On both MR and CT, the imaging characteristics may be altered by intracystic hemorrhage. There is variable enhancement of cyst septations on both MR and CT (see Fig. 9 ). In both modalities, microcystic LMs often have some degree of heterogeneous enhancement and ill-defined borders.
Diagnosis: imaging characteristics
Low-flow vascular malformations can often be correctly diagnosed based on physical examination and history. Anomalous draining veins of venous malformations can on occasion be seen superficially, and VMs tend to be compressible, have a characteristic bluish color, and to expand when venous drainage is slowed. Lymphatic malformations are soft, noncompressible translucent masses with overlying normal or bluish skin, often with superficial vesicles (in microcystic cases). Nevertheless, imaging plays an essential role in characterizing these lesions to confirm the diagnosis (such as excluding the diagnosis of neoplasm), to define the spatial extent of the lesion and its relationship to important nearby structures, and for treatment planning.
Superficial and small lesions are well examined by ultrasound, with gray scale studies defining the extent and compressibility of the lesion, and spectral and color Doppler interrogation used to identify the flow characteristics. Sonography can also play a crucial role in differentiating macrocystic and microcystic lymphatic malformation ( Fig. 6 D). Very superficial lesions may be remarkably inconspicuous on MR imaging and yet well defined on ultrasound. Additionally, ultrasound has significant utility in providing image guidance for sclerotherapy, as described below.
However, in most cases, we have found MR imaging to be virtually indispensible. Its unparalleled contrast resolution more reliably determines the full extent of the lesion than any other modality. We most heavily rely on pre- and post-contrast T1-weighted images as well as T2-weighted images, and consider fat suppression to be critical in all MR sequences in the workup of vascular anomalies (see Fig. 6 ). Other MR techniques, such as MR angiography, venography, and lymphangiography, tend to contribute little in cases of low-flow anomalies. CT plays more of an adjunctive role in the workup of vascular malformations, being of most use in cases where bony involvement is crucial to delineate (as in sinus, skull base, cranium, or mandibular cases).
Untreated, both venous and lymphatic malformations typically appear hyperintense on T2 imaging, and the scope of the abnormal T2 signal can be used to define the overall extent of the lesion. After treatment by sclerotherapy, with the resultant progressive conversion of lesion to scar tissue, the T2 signal characteristically becomes less hyperintense. The enhancement pattern after contrast administration is a crucial differentiating feature, with venous malformations most characteristically enhancing avidly but in a patchy, heterogeneous pattern (see Figs. 2 to 4 ). In contrast, enhancement of lymphatic malformations is variable (see Fig. 6 ). Mild rim enhancement may be seen with macrocystic lymphatic malformations, with only minimal enhancement identified in microcystic LMs ( Fig. 7 ); however, in the setting of superinfection or inflammation, LMs may enhance avidly as well.