Fig. 2.1
Algorithmic approach to Imaging for patients presenting with headache
Seizure
A seizure is a finite event of altered cerebral function because of excessive and abnormal electrical activity within the brain. Epilepsy is a chronic condition which predisposes the patient to repeated seizures. It has been estimated that one out of eight individuals will experience at least one seizure in their lifetime [22].
The International League Against Epilepsy reclassified epileptic seizures in 2010 in an effort to improve diagnosis, treatment, and management of patients who live with this chronic illness (Fig. 2.2). They classified seizures as generalized or focal and then further subdivided them into tonic-clonic, absence, myoclonic, clonic, tonic, and atonic types. The distinction between “focal” and “generalized” seizures is important because it reflects the extent of abnormal brain activity. Generalized seizures rapidly affect both cerebral hemispheres and both sides of the body, even when they are caused by a “focal” lesion. Certain types of seizure disorders are more likely than others to be associated with structural brain lesions such as tumors, infection, infarction, traumatic brain injury, vascular malformations, and developmental abnormalities. Therefore, knowledge of the type of a patient’s seizure helps to determine when neuroimaging is clinically indicated and what type of study is appropriate.
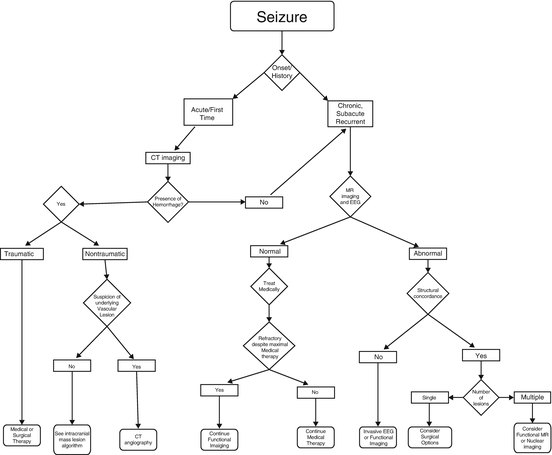

Fig. 2.2
Algorithmic approach to imaging for patients presenting with seizure
Given the superior soft tissue contrast and multi-planar capability of MRI, it is easy to understand why it provides the highest sensitivity and accuracy when assessing virtually any cause of epilepsy. MR can provide high-resolution structural imaging in epilepsy with either 1.5 or 3.0 T MR scanners, including tailored thin section imaging through the mesial temporal lobes in cases of temporal lobe epilepsy (TLE). In these patients, MR imaging can demonstrate hippocampal atrophy, subtle signal alterations, certain structural abnormalities accompanying cortical dysplasias, hamartomas, and / or other developmental abnormalities. Anatomic imaging identifies a focal abnormality in as many as half of all patients with focal seizures [23].
Despite the technical advances, morphologic imaging fails to reveal a structural abnormality in a great number of patients who suffer from refractory seizures. In these patients, functional studies can provide useful information on the source of the seizure. Functional imaging techniques include PET, single-photon emission computed tomography (SPECT), magnetic source imaging (MSI), and functional MRI (fMRI). These techniques should be employed in the evaluation of patients with epilepsy who are candidates for surgical intervention [24, 25–31].
Clinical PET with fluorine-18-2-fluoro-2-deoxy-d-glucose (FDG) provides a measure of glucose uptake by brain cells and therefore brain metabolism. A seizure focus typically manifests as a focus of hypometabolism on interictal examinations. Ictal imaging, however, will show increased glucose metabolism and therefore increased FDG activity on PET imaging. When compared with EEG results, interictal FDG-PET is sensitive (84 %) and specific (86 %) for TLE. On the other hand, it is much less sensitive but more specific. By contrast, structural-based temporal lobe MR imaging yields lower sensitivity than PET imaging. Outside of the the temporal lobe, MR imaging yields higher sensitivity but lower specificity than PET imaging. Therefore, a combination of EEG, MR, and PET imaging may be prudent in certain seizure foci prior to surgical intervention.
Both contrast enhanced MR and SPECT, using perfusion agents such as 99mTc-HMPAO or 99mTc-Neurolite, provide an assessment of regional cerebral blood flow (CBF) rather than brain metabolism. Similar to metabolic imaging, perfusion imaging shows hypoperfusion during interictal imaging and hyperperfusion during ictal imaging. The use of ictal/interictal subtraction imaging with co-registration on MRI and image-guided surgery datasets is proving to be more useful than interictal imaging alone [32, 33]. Unfortunately, the need to inject the blood flow agent within 90 seconds of the seizure for the ictal phase of imaging is often an insurmountable technological challenge.
fMRI techniques include phosphorus and proton spectroscopy (MRS), perfusion, and blood oxygen level-dependent (BOLD) activation. Widespread availability of fMRI imaging is limited because this technique requires specialized MR scanners that can perform and then post-process fast echo-planar pulse sequences. MRS provides in-vivo chemical analysis of the brain. It shows differential metabolite values in epileptogenic regions of the brain compared to normal brain parenchyma. MRS is used as an adjunct pre-surgical examination for seizure source localization in difficult cases of extra-temporal and partial epilepsy. It reduces the need for invasive intracranial electroencephalography (iEEG) recordings.
Both EEG and magnetoencephalography (MEG) offer significantly higher temporal resolution than PET, SPECT, and fMRI. Recent improvements in MEG technology allow complete brain coverage. MEG is utilized in preoperative evaluation of patients with intractable or medically refractory seizures [34, 35]. The MEG images are often superimposed on high-resolution MR images. MEG is not a “frontline” tool for evaluation of epilepsy, but may be used in select patients who: (a) are surgical candidates for resection, (b) do not have an MR demonstrable lesion or have multiple potential seizure foci, or (c) might otherwise require invasive monitoring (iEEG). MEG is thus complimentary to EEG and may confirm lesions seen on MRI as the source of the seizure. MEG provides better spatial resolution compared with EEG [35] and can also guide the placement of iEEG grids. In certain patients it may also help to discern important seizure foci among multiple potential seizure foci suggested by other tests.
Focal Neurologic Deficit
A focal neurologic deficit is a collection of symptoms or signs that can be attributed to a specific anatomic site in the CNS. A dedicated neurologic examination often can define the CNS source of the deficit. In some cases, however, the etiology of the abnormality may be difficult to define, and in these cases imaging can narrow differential diagnostic considerations. Image interpretation can be much more sensitive and specific if the radiologist is made aware of the onset (acute vs. insidious) as well as temporal behavior (stable, worsening, or resolving) of the focal neurologic deficit.
In general, acute onset of a focal neurologic deficit implies a vascular etiology while a more insidious onset or a chronic process suggests an underlying mass lesion (Fig. 2.3). CT imaging can be used to screen patients for suspected infarction, but is often insensitive in the hyper-acute setting. At times, certain subtle CT findings such as an obscured insular ribbon or hyperattenuation of the middle cerebral artery can be the first imaging clue to the location as well as severity of the stroke on early CT. Diffusion weighted MR imaging (DWI) is much more sensitive than CT in detecting acute ischemia-induced cytotoxic edema and thus the specific brain tissue at risk. The main goal of CT in the setting of acute infarction is to exclude intracranial hemorrhage in patients who might otherwise be candidates for thrombolytic therapy. Contrast administration is not necessary with either CT or MR when evaluating for acute infarction.
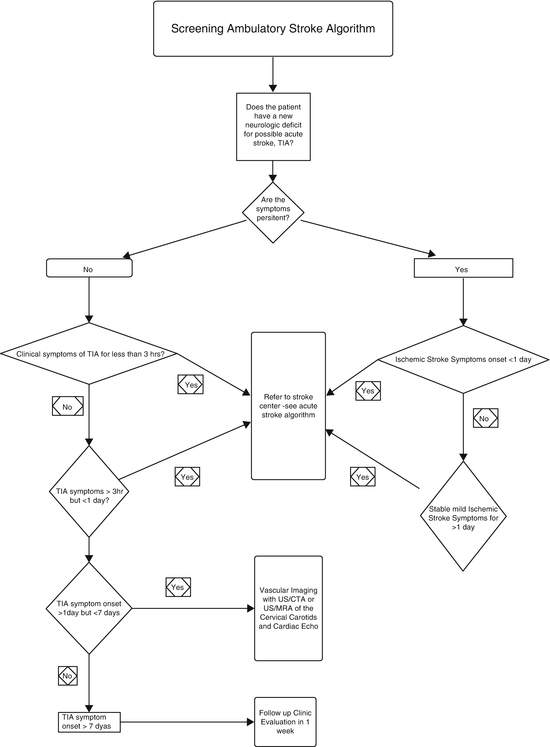

Fig. 2.3
Algorithmic approach to imaging patients who present with a focal neurologic deficit in the ambulatory setting: screening for acute stroke
Besides ischemic events, parenchymal or SAH also may cause an acute focal neurologic deficit. Non-contrast CT imaging is the preferred technique for screening for acute intracranial hemorrhage because of wide availability, short scan time, and high sensitivity for detecting acute hemorrhage [36, 37]. While MR is sensitive for detecting and differentiating acute from chronic blood products, it is generally not as available in the acute setting.
A slowly progressive focal neurologic deficit generally implies the presence of an expanding and evolving intracranial lesion such as a primary or metastatic neoplasm. Conversely, a more subacute presentation typically correlates with an infectious process. While CT is invaluable for screening for either infection or neoplasm acutely, a contrast enhanced MR study will provide far more anatomic detail, define the extent of disease, and allow for soft tissue characterization.
Transient Ischemic Attack
Over the past decade, there has been considerable debate over the definition of transient ischemic attack (TIA), particularly the acceptable duration of the transient symptomatology. Conventionally, TIA was defined as a focal neurologic deficit lasting less than 24 h. The problem with a time-based definition is that it does not adequately identify patients who may benefit from thrombolytic therapy (rtPA, recombinant tissue plasminogen activator). A tissue-based definition is preferable because although most (70 %) TIA symptoms last for 2 h or less, many of these patients (30–50 %) show tissue injury on diffusion weighted MRI (DWI) [38–40]. The American Stroke Association has recently proposed a new definition of TIA as “a transient episode of neurologic dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction” [39, 41]. Based on the current FDA recommendations, however, only the presence of acute hemorrhage on non-contrast CT (NCCT) is a contraindication to rtPA treatment in the first 3 h after the onset of deficit. Even rapidly improving symptoms in the first 3 h and no DWI changes on MR may not justify withholding rtPA because as many as a third of these patients go on to subsequent severe deterioration if left untreated.
In addition, 10–15 % of all strokes are heralded by a TIA within 90 days—half of these within 48 h. Thus, a history of recent TIA should trigger an immediate workup for stroke risks and additional tissue and vascular imaging studies [39, 42, 43]. These imaging studies typically begin with non-contrast CT, followed by MR and carotid Doppler US to identify a possible source of thromboembolic disease.
Stroke
As noted above, NCCT has been the preferred modality for initial imaging of suspected stroke because it is widely available and very sensitive for acute hemorrhage. CT effectively detects acute hemorrhage in brain parenchyma and in the subarachnoid, subdural, and intraventricular spaces. Unfortunately, it is insensitive at detecting acute ischemic tissue injury. Recent development of computed tomographic perfusion (CTP) imaging has increased sensitivity for detection of acute cerebrovascular-induced tissue damage. While CTP imaging requires contrast, magnetic resonance perfusion (MRP) imaging may be performed with or without contrast.
CT perfusion studies are performed by rapidly imaging and reimaging a target region of the brain immediately following contrast bolus. The technique is based on the central volume principle which states that CBF is equivalent to the cerebral blood volume (CBV) divided by the mean transit time (MTT). Both CTP and MRP imaging can measure CBV, CBF, time to peak (TTP), and MTT. Quantitative and qualitative measurement of these parameters provides a method of assessing regional tissue perfusion to identify relative regions of ischemia or infarction. In general, a CBF of 10–15 mL/100 g/min or less is considered infarcted tissue whereas a rate of 15–20 mL/100 g/min is considered ischemic. Any region of tissue that is under-perfused, but not infracted, surrounding infracted tissue is considered the penumbra. Alternatively the penumbra can also be considered as a volume of low blood flow tissue that is larger than the infarcted volume by 20 % or more. Relative “mismatch” of tissue flow and volume compared with the centrally infracted core tissue, in the absence of acute hemorrhage, indicates brain tissue that may be salvaged with the use of thrombolytic therapy. Technically, MRP has several advantages over CTP. It does not use ionizing radiation, has less risk of renal toxicity, known contrast reactions, and does not cause fluid overload compared to iodinated contrast materials. MRP has less variability than CTP quantitative methods [44], and, unlike CT, MRI can measure directly cellular viability using diffusion restriction techniques. It also can assess a larger volume of brain tissue than CTP. The newer generation of CT scanners, with their larger number of detector arrays, is decreasing the volume gap between techniques, however [45]. Despite these advantages, the wide availability of CT and the rapidity with which it can be performed in a limited 3 h clinical onset window mitigates the advantages of MR. Given the abovementioned risks and benefits of imaging, an appropriate plan of care can be rapidly implemented to diagnose, define, and possibly treat a patient presenting with an acute stroke (Fig. 2.4).
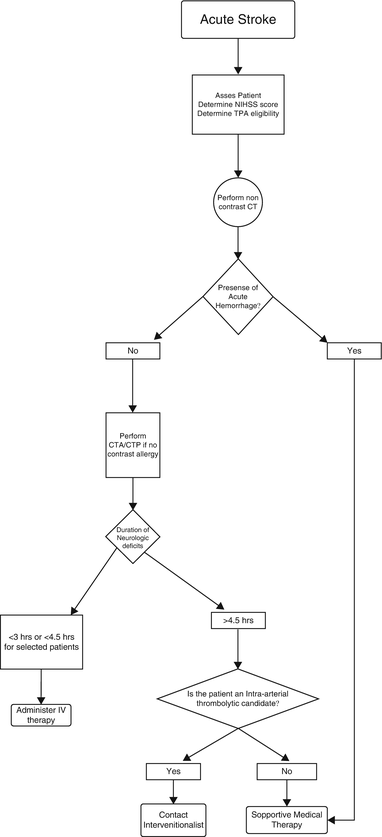

Fig. 2.4
Algorithmic approach for imaging and treatment of acute stroke
Role of Imaging in Specific Scenarios
Carotid Stenosis
In recent years, the medical community has made a concerted effort to reduce the incidence of stroke by identifying patients most at risk. Much effort has been expended to identify risk factors for atherosclerotic disease, and new strategies have been developed to reduce the rate of stroke in high-risk patients [46]. Efforts range from modification of lifestyle to preemptive surgical or endovascular carotid artery intervention. Randomized, prospective clinical trials that include imaging and other criteria have shown that surgical endarterectomy is effective in reducing stroke morbidity of both asymptomatic and symptomatic patients [47–52].
Imaging today offers a number of sensitive, noninvasive and therefore low risk, tests, all directed at diagnosing the most common cause of a stroke—carotid artery atherosclerosis—in “at risk” patient groups such as those with a carotid bruit [53, 54]. While duplex ultrasound (US) and computed tomography angiography (CTA), magnetic resonance angiography (MRA) and time-resolved contrast enhanced MRA (CE-MRA) all have high diagnostic accuracies for detecting internal carotid stenosis, 70–99 % [55, 56], only US appears to offer cost-effective initial screening. However, given the operator imaging variability with sonography as well as the artifact created from calcified plaques, and the difficulty in distinguishing subtotal from total occlusion, a full endorsement of its routine use as the sole examination before endarterectomy cannot be made [55, 57]. Most radiologists today recommend combined use of US with CE-MRA [53–55, 57–59].
Multi-slice CTA offers an alternative means of examination; however, it poses risks from intravenous iodinated contrast administration. CTA requires a large intravenous contrast injection volume and hence has the potential for contrast-induced nephrotoxicity or anaphylaxis. In addition, CT exposes the patient to an ionizing radiation dose. CTA also may be less accurate at evaluating the degree of luminal narrowing in the presence of calcified plaque than some other techniques [56, 60, 61].
It is hoped that better plaque characterization will improve the predictive value of imaging at identifying clinically significant carotid stenosis for patients with symptomatic cerebral ischemia [62, 63]. A variety of imaging strategies could be undertaken in symptomatic patients at risk for major ischemic stroke, where the initial study could include a brain imaging examination, quickly followed by one of the abovementioned noninvasive vascular studies.
In patients with chronic carotid stenosis or occlusion, the elevated ischemic stroke risk could be further evaluated by functional imaging. With perfusion imaging, CBV and CBF can be quantitatively and qualitatively assessed. In the recent past, this was performed with a nuclear SPECT flow study during a rest and “challenge” phase following the administration of Acetazolamide. This same principle also can be utilized with 15O-PET where the oxygen extraction differences pre- and post-challenge permit calculation of cerebral vascular reserve (CVR) [64–66]. It has been shown that CVR inversely correlates with stroke risk. Low CBV measurement by either CT or MR appears to correlate with reduced CVR and increased stroke risk [67, 68]. Compared with other examinations, CT and MR are becoming more widely accepted for functional assessment of the at-risk brain tissue because they are more widely available than PET, have better resolution than SPECT, and have overall shorter study times compared to nuclear imaging in general.
Triaging Patients for Thrombolytic therapy
Since the advent of reperfusion therapies, acute ischemic stroke has been transformed from incurable and nonurgent to most emergent and critically treatable. As discussed above, the ability to treat acutely ischemic tissue has impelled physicians to develop a method by which they could distinguish viable from nonviable brain parenchyma.
Current clinical practice in the United States is based on the 1996 FDA approval of intravenous (IV) rtPA therapy. Once cerebral hemorrhage has been excluded using NCCT, the drug is administered, preferably within 1 h and no later than 3 h after symptom onset. As a part of accepted protocol, acute stroke must obtain the NCCT within 25 min of admission and expert interpretation within the next 20 min (45 min “door-to-interpretation” time) [69]. Recent increases in public awareness, faster emergency medical response, and establishment of dedicated stroke centers have resulted in 19–60 % of admissions arriving at treatment centers within 3 h of symptom onset.
Despite these efforts, only 3–8.5 % of ischemic stroke admissions qualify for rtPA therapy [70–72]. Because of the small numbers of acute stroke patients qualifying for treatment within the current 3-h limit, interest in expanding the treatment window has been growing if it can be shown not to increase the risk of hemorrhage. A pooled risk-benefit analysis of existing rtPA trials using NCCT scan for the exclusion of hemorrhage has suggested that rtPA may be safe in some patients out to 4.5 h after stroke, but the FDA and ASA recommendations have not yet been modified to include this expanded treatment window in published guidelines. The American College of Radiology (ACR) appropriateness criteria may change in the very near future as the results of these studies and trials become conclusive.
There is growing evidence that intra-arterial (IA) thrombolytic delivery and mechanical clot extraction methods are beneficial either alone or with IV rtPA therapy in patients who fall outside the 3-h limit or who have large-vessel occlusion or larger clot burden. They may be at a higher risk of hemorrhage, however. Furthermore, complexities of organizing these later stage therapies have limited their widespread adoption in general [73–75, 76].
Many centers now routinely perform CT perfusion, but currently there is no clear consensus on whether the information obtained from CTP should be used to guide intravenous (IV) or intra-arterial (IA) therapy. MR perfusion and diffusion imaging could be performed instead, but unfortunately this technique is time-prohibitive and not universally available on a 24 h/7days a week basis. Furthermore, contraindications listed previously (Table 1.3, Chap. 1) may preclude patients from having an MR.
It should be noted that in addition to NCCT to exclude acute hemorrhage in new onset stroke patients, previously described multimodality MRI and CT studies also may be useful to confirm the diagnosis, classify the subtype of stroke, demonstrate lesion location, identify vascular occlusion, and guide other management decisions both within and beyond the 3 h period. Currently, however, ASA and others’ guidelines specifically recommend that emergency IV rtPA treatment within the 3 h window not be delayed in order to obtain multimodality imaging studies and furthermore that treatment not be withheld on the basis of either negative or additional positive MR or CT findings, other than acute hemorrhage [77–81].
Intracranial Mass Lesion
While the discovery of an intracranial mass lesion may be incidental, patients can present with focal or non-focal neurologic deficits. Signs or symptoms may range from headaches, seizures, syncope, to change in mental status. Clinical history along with imaging can provide a framework for proper categorization of the disease process. General categories for possible etiologies of an intracranial mass lesion include infectious, inflammatory, traumatic, vascular, or neoplastic. Contrast utilization is often quite helpful not only in characterizing the disease process but also evaluating the full anatomical extent of disease. While the role of imaging initially was limited to providing anatomic details, the present role is to provide functional information through a vast array of modalities such as perfusion, diffusion, MR spectroscopy, and PET [82]. Sophisticated imaging techniques allow insight into such processes as the free movement of water molecules, microvascular integrity, chemical composition, and glucose metabolism of the lesion. Merging the morphologic and functional information gained from these advanced imaging techniques can then provide a more comprehensive assessment of the lesion so that we may better understand its physiology and in turn predict its behavior (Fig. 2.5).
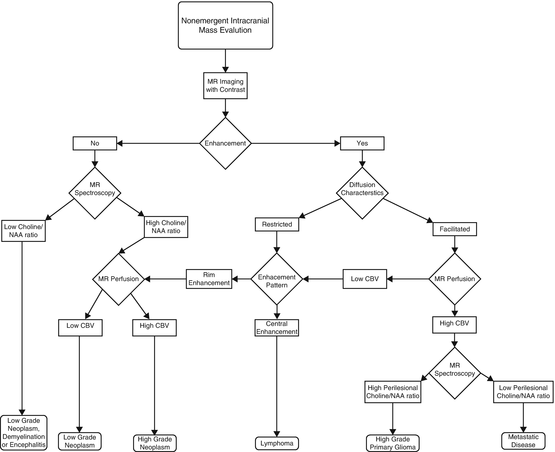

Fig. 2.5
Algorithmic approach for image-based characterization of an intracranial mass lesion
Subarachnoid Hemorrhage
Since CT is highly accurate for the diagnosis of acute hemorrhage, it has been the mainstay in emergent evaluation of acute intracranial bleeding, especially subarachnoid or parenchymal hemorrhage, both of which are associated with high morbidity and mortality [83, 84]. In the case of SAH caused by aneurysms, the high morbidity rate is partly due to a high likelihood of re-bleeding. Early surgical intervention or intravascular coiling is recommended to reduce morbidity. Before therapy can be undertaken, a cerebral angiogram is required to define the aneurysm’s location and morphology. Studies show that catheter-based angiography has approximately 90 % sensitivity in detecting an aneurysm. This sensitivity, however, decreases to approximately 80 % in the setting of SAH resulting from small aneurysm size or in the face of aneurysm thrombosis, local vasospasm, or an incomplete study [85, 86]. Traditionally, non-visualization of an aneurysm on the initial scan from any of the above reasons warranted a follow-up catheter-based repeat angiogram 1 week after the initial examination. The clinician should be aware that the cost and risk to obtain the additional 1–2 % diagnostic yield has been debated [87].
Today, clinical practice has shifted toward initial NCCT for SAH detection and if found immediate CTA for aneurysm detection. Studies have shown that CTA has overall detection sensitivities of 85–95 % for aneurysms greater than 2 mm in diameter [88–90]. Today, treatment of intracranial aneurysms following SAH is increasingly based on CTA alone [91, 92]. In addition, the appearance of new neurologic changes suggestive of post-SAH vasospasm, ischemia, or hydrocephalus is increasingly investigated with transcranial Doppler (TCD) and CT imaging with CTA and CTP. Catheter angiography and 123I-IMP SPECT are used less frequently than in the past [85, 93–98].
Aneurysm Screening
In the absence of predisposing risk factors such as family history, polycystic kidney disease, connective tissue disorder, or collagen vascular disease, the need to screen the general population for aneurysms is debated. Proponents raise concerns over the cumulative long-term risk of morbidity and mortality from SAH, particularly for aneurysms larger than 2.5 cm. When this is weighed against the relatively low risk of clipping and coiling unruptured intracranial aneurysms, data suggest that there may be a clinical role for prophylactic aneurysm screening [99, 100




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree