(1)
Princess Elizabeth Hospital Le Vauquiedor St. Martin’s Guernsey, Channel Islands, UK
(2)
Brighton and Sussex Medical School, Brighton, England UK
Abstract
Pseudoangiomatous stromal hyperplasia (PASH) was first described by Vuitch et al. in 1986. The authors described the tumorous, histological and ultrastructural features of PASH in nine premenopausal women. The women presented with unilateral breast masses. PASH is a benign proliferative lesion of the stroma whose aetiology and pathogenesis are ill-understood (Virk and Khan 2010). Leon et al. (2002) believed the proliferation originated from mammary fibroblasts and proposed the term nodular myofibroblast hyperplasia of the mammary stroma which indicates its true histogenesis. The stromal hyperplasia in PASH is due to exaggerated, aberrant responsiveness of the mammary myofibroblasts to hormonal stimuli, either endogenous or exogenous. The main hormone implicated is progesterone. The nuclei in PASH are progesterone receptor (PR) positive, whereas expression of oestrogen receptors (ER) is variable (Anderson et al. 1991). Powell et al. (1995) also reported expression of PR in 36 % (5/14) and ER in 12 % (2/14) cases of PASH. Further hormonal basis for this lesion is supported by the fact that half of postmenopausal women with tumorous PASH were on hormone replacement therapy (HRT) and occurred in men with gynaecomastia (Powell et al. 1995; Anderson 1991). The origin of the slit-like spaces is unknown but on electron microscopy the spaces are lined by an incomplete layer of spindle cells joined through cell junctions or occasionally by tight junctions (Vuitch et al. 1986).
Learning Points
PASH is a benign localised mesenchymal/stromal proliferative lesion with possible hormonal aetiology.
PASH is characterised by slit-like spaces resembling capillaries within a dense fibrous stroma.
PASH cells show myofibroblastic differentiation and express progesterone receptors.
PASH has an excellent prognosis and in most cases local excision is sufficient.
Fat necrosis of the breast is usually due to trauma.
Presence of fibrosis in fat necrosis with associated architecture distortion can simulate malignancy.
Cyst formation, calcification and haemosiderin deposition can occur at a later stage in fat necrosis.
Localised fibrosis often presents as a non-calcified mass on radiological imaging and the diagnosis should be accepted on needle core biopsy if there is radiological concordance.
MRI is useful in assessing postsurgical scarring to exclude malignancy.
Haemosiderin deposition can mimic calcification mammographically.
14.1 Pseudoangiomatous Stromal Hyperplasia
14.1.1 Aetiology and Pathogenesis of PASH
Pseudoangiomatous stromal hyperplasia (PASH) was first described by Vuitch et al. in 1986. The authors described the tumorous, histological and ultrastructural features of PASH in nine premenopausal women. The women presented with unilateral breast masses. PASH is a benign proliferative lesion of the stroma whose aetiology and pathogenesis are ill-understood (Virk and Khan 2010). Leon et al. (2002) believed the proliferation originated from mammary fibroblasts and proposed the term nodular myofibroblast hyperplasia of the mammary stroma which indicates its true histogenesis. The stromal hyperplasia in PASH is due to exaggerated, aberrant responsiveness of the mammary myofibroblasts to hormonal stimuli, either endogenous or exogenous. The main hormone implicated is progesterone. The nuclei in PASH are progesterone receptor (PR) positive, whereas expression of oestrogen receptors (ER) is variable (Anderson et al. 1991). Powell et al. (1995) also reported expression of PR in 36 % (5/14) and ER in 12 % (2/14) cases of PASH. Further hormonal basis for this lesion is supported by the fact that half of postmenopausal women with tumorous PASH were on hormone replacement therapy (HRT) and occurred in men with gynaecomastia (Powell et al. 1995; Anderson et al. 1991). The origin of the slit-like spaces is unknown but on electron microscopy the spaces are lined by an incomplete layer of spindle cells joined through cell junctions or occasionally by tight junctions (Vuitch et al. 1986).
14.1.2 Clinical Features of PASH
PASH can affect any women between the age of 12 and 75 years, but with a predominance in postmenopausal women (Vuitch et al. 1986; Powell et al. 1995; Ferreira et al. 2008). PASH is also seen in men with gynaecomastia (Milaneze et al. 1998). PASH may be detected as an incidental finding on microscopic examination or palpable slow growing breast mass. On examination tumorous PASH is a solitary firm, painless mass mimicking a fibroadenoma but may also enlarge rapidly to mimic a malignant tumour with bilateral diffuse enlargement of the breasts (Yoo et al. 2007). The differential diagnoses of PASH include low-grade angiosarcoma, myofibroblastoma, fibroadenoma and mammary hamartoma (Virk and Khan 2010).
14.1.3 Radiological Features of PASH
There are no specific radiological features for PASH. Mammographically PASH consists of a well-circumscribed round to oval density without calcification (Polger et al. 1996). On ultrasonography, PASH exhibits a solid, well-circumscribed, homogenous, hypoechoic mass; the mass may be ill-defined or hyperechoic. MR imaging reveals isointense mass on T1-weighted gradient echo images and may show linear reticular ‘lace-like’ pattern on axial T2-weighted images due to slit-like spaces in PASH (Mercado et al. 2004; Yoo et al. 2007).
14.1.4 Pathological Features of PASH
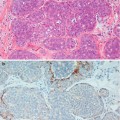
Tumorous PASH varies in size from 0.6 mm to 12 cm (Powell et al. 1995; Ferreira et al. 2008; Polger et al. 1996). Sasaki et al. (2008) documented a 20 cm mass in a 36 year old woman. Macroscopically tumorous PASH is round to oval, well-circumscribed with a rubbery texture. The outer surface is smooth and un-encapsulated. The cut surface has a homogenous solid appearance with a grey white appearance and occasional cysts (Mercado et al. 2004). Histologically, PASH is characterised by dense fibrous stroma separated by complex anastomosing of slit-like spaces (Fig. 14.1). The spaces are lined by discontinuous spindle cells. The stroma lacks cytological atypia and there is no mitotic activity. The epithelium of the lobules and ducts may be unremarkable or may show ductal epithelial hyperplasia with apocrine metaplasia (Virk and Khan 2010). In gynaecomastia, there may be associated epithelial proliferative change. Needle core biopsies have been reported to have 83 % sensitivity in diagnosing PASH (Wieman et al. 2008). Cytology is not helpful in making a diagnosis of PASH (Virk and Khan 2010).
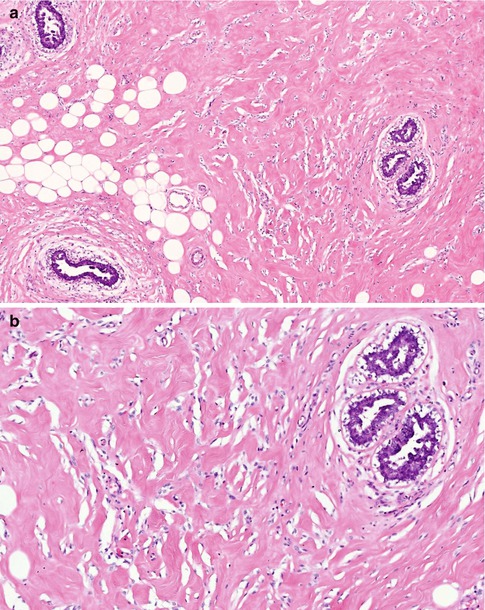

Fig. 14.1
(a) PASH is a common finding in gynaecomastia; arbotive male breast lobules are surrounded by dense hyalinised stroma with numerous slit-like spaces. (b) At high magnification, the slit-like spaces are irregular, resemble capillaries but without red blood cells. The slit-like spaces are partly lined by discontinuous spindle cells
On immunocytochemistry, the spindle cells lining the slit-like spaces are positive for myofibroblastic markers, CD34, Vimentin and smooth muscle actin (SMA) (Vuitch et al. 1986; Powell et al. 1995). The cells are negative for cytokeratin, S100 protein and endothelial markers such as Von Willebrand factor antigen and CD31.
14.1.5 Management of PASH
There is no additional specific treatment if PASH is found incidentally in specimens excised for other lesions. Excision with adequate close margin is recommended treatment for tumorous PASH. Diffuse PASH may require wide excision of the breast or mastectomy for cosmetic reasons or for persistent pain and discomfort (Virk and Khan 2010). The recurrence rate after excision ranges from 0 % to 22 % (Powell et al. 1995; Ferreira et al. 2008; Wieman et al. 2008). The recurrence can occur in the ipsilateral or contralateral breast. There is no established medical treatment for PASH, although tamoxifen has been reported to have induced complete resolution of bilateral PASH (Pruthi et al. 2001). Overall the prognosis of PASH is excellent and is not considered a premalignant lesion or risk factor for malignancy (Virk and Khan 2010).
14.2 Fat Necrosis
14.2.1 Pathogenesis of Fat Necrosis
Mammary fat necrosis is an important benign lesion because of its ability to mimic cancer both clinically and mammographically. Fat necrosis is associated with trauma, especially in women with pendulous breasts. However, a history of trauma is present in only up to 65 % of cases (Bilgen et al. 2001). Other causes of fat necrosis include seat-belt trauma, cyst aspiration, needle core biopsy, lumpectomy, radiation therapy, reduction mammoplasty, breast reconstruction with transverse rectus abdominis myocutaneous flap and removal of implant and anticoagulant therapy (Hogge et al. 1995; DiPiro et al. 1995). Microscopically, fat necrosis is caused by sterile inflammation resulting from leakage of fatty acids from the adipocytes, leading to a polymorph, lymphocytic and macrophage infiltrate with associated giant cell reaction. Saponification by blood and tissue lipases leads to formation of vacuoles surrounded by macrophages. Clinically, fat necrosis can present with a firm and fixed lump, with or without skin or nipple retraction. Lanyi (1986) succinctly illustrated stages in the pathogenesis of fat necrosis as follows:
1.
Lesion phase – damage to the fat cells with leakage of the neural fat.
2.
Absorption phase – lipophages remove the liberated neutral fat leading to formation of fat vacuoles with macrophage, plasma cell and other inflammatory cell infiltrate.
3.
Repair phase – increasing numbers of fibroblasts follow the absorption phase, walling off a cavity with a dense capsule or scar formation. Calcium salts or haemosiderin deposition may occur in the fibrosis. The calcification of the oil cyst may exhibit an eggshell appearance.
14.2.2 Radiological Features of Fat Necrosis
Fat necrosis is not an uncommon lesion. In Lanyi’s series (1986) of 1,044 consecutive mammograms, he identified 90 liponecrotic microcysts, which constituted 8.6 % of the mammographic lesions. The mammographic appearance of the fat necrosis depends on the stage of the lesion. In the early stages, fat necrosis produces an ill-defined radiolucent mass (Heywang-Köbrunner et al. 2001).
When liponecrotic cysts develop, they are usually microcysts and rarely macrocysts (Lanyi 1986). The microcysts are usually 2–3 mm and exhibit a central lucency surrounded by punctate or amorphous calcification of the peripheral rim. Frequently the cysts are solitary but multiple clusters can also occur. These lesions are difficult to examine histologically because the calcium shatters under the microtome blade.
Calcification in fat necrosis can appear pleomorphic and clustered. Mammographic magnification may be necessary to highlight some ring-forms, which are features of benignity (Heywang-Köbrunner et al. 2001). Branching, rod-like or angular microcalcifications in fat necrosis may be indistinguishable from carcinoma (Bassett et al. 1978). Ruptured cysts in fibrocystic change and duct ectasia also cause fat necrosis with excessive scarring. Fat necrosis can produce stellate or spiculate lesions, which again closely resemble carcinoma (Meyer et al. 1978).
DiPiro et al. (1995) followed up five women who sustained fat necrosis from seat-belt injuries. At 1–2 months following the breast injury, the mammograms showed thin-walled fat-density cysts in a linear distribution, and in less dense breasts there was an associated 2–3 cm band of increased density. The latter was not apparent in more dense breasts. By 3–4 months after the injury, the lipid cysts and contusions were less apparent and a line of fibrosis had developed. On ultrasound, the lipid cysts were smoothly marginated and anechoic or hypoechoic. Parenchymal calcification may develop 3.5–4 years later.
In a larger study, Bilgen et al. (2001




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree