The superior tissue contrast and flexible imaging planes afforded by magnetic resonance imaging (MRI) versus competing technologies permit optimal depiction of the pelvic viscera. Targeted protocols developed for specific pelvic visceral organs highlight important anatomic features that may not be imaged by other modalities. Therefore, a solid understanding of normal and variant pelvic anatomy is crucial for appropriate interpretation of pelvic MRI studies. This article discusses the protocol strategies and relevant anatomy with commonly encountered anatomical variants in a segmented/organ-specific manner, using gender as a broad split given the substantial variance in relevant organs.
Because of its superior tissue contrast, native multiplanar capabilities, and nonionizing technique, magnetic resonance imaging (MRI) is well suited for evaluation of the pelvis. Although ultrasound is frequently indicated for the primary evaluation of suspected genitourinary pathology in both men and women, it can be limited by body habitus, depth of acoustic penetration, and ability to discriminate between specific tissue types. Knowledge of normal pelvic anatomy on MRI is critical for proper interpretation, in particular the standard visceral organ appearances, commonly encountered variants, and pathology mimics. Because most pelvic MRI studies are targeted toward an organ or area of interest, this article discusses the protocol strategies and relevant anatomy in a segmented/organ-specific manner, using gender as a broad split given the substantial variance in relevant organs.
Female pelvis
Technical Considerations
At the authors’ institution, the female pelvis protocol is performed for suspected ovarian, uterine, vaginal, or bladder pathology; a separate protocol is used for high-resolution imaging of the female urethra. Studies are performed using a phased-array surface coil and intramuscular injection of an antiperistaltic agent to suppress bowel motion, which improves visualization of the adnexal and peritoneal surfaces.
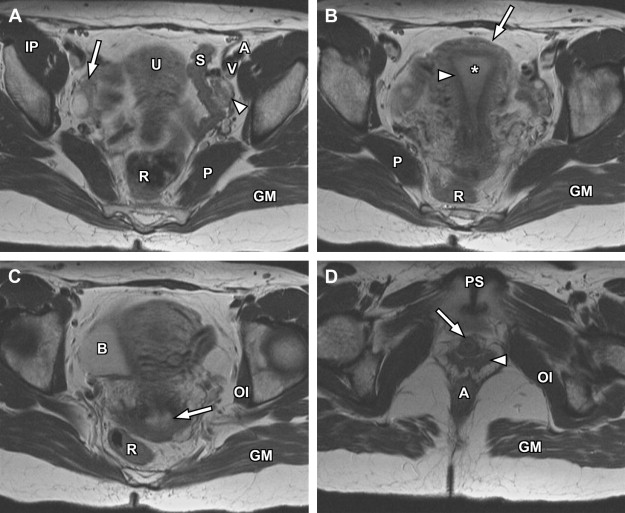
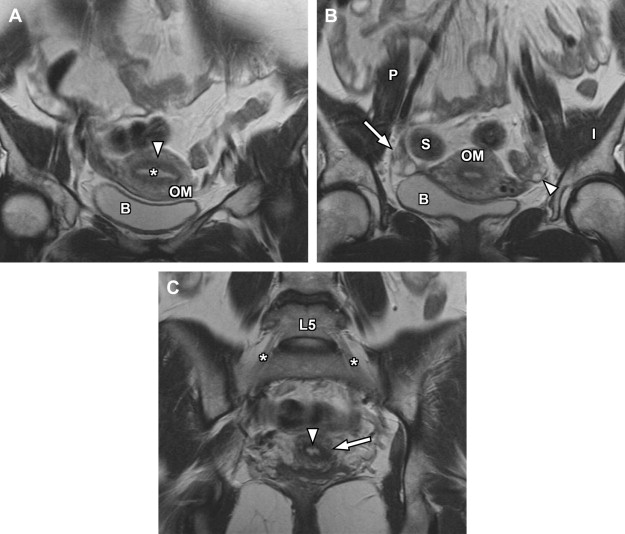
The basic female pelvis protocol begins with a coronal T2-weighted single-shot echo-train spin-echo technique (such as single-shot fast spin-echo [SSFSE] or half-Fourier acquisition single-shot turbo spin-echo [HASTE]), with wide field of view (FOV) to include portions of the kidneys, due to the coexistent risk of renal anomalies in the setting of suspected genitourinary developmental disorders. Subsequently, a sagittal T2-weighted fast spin-echo sequence is used to delineate the uterine lie, demonstrate the zonal anatomy of the uterus, and provide improved anatomic landmarks for subsequent uterine image planning ( Fig. 1 ). Both short-axis and long-axis T2-weighted fast spin-echo images are then obtained to provide redundant detail of the uterine zonal anatomy and optimize evaluation of the external uterine contour ( Figs. 2 and 3 ). T1-weighted imaging is initially accomplished using a breath-hold spoiled gradient-echo sequence in which both in-phase and opposed-phase echoes are recorded ( Figs. 4–6 ). Subsequently, T1-weighted imaging using chemical fat suppression is performed, which aids in the differentiation of lipid and hemorrhagic/proteinaceous pathologies on T1-weighted imaging. Additional postcontrast images are obtained using the same sequence (see Figs. 4–6 ). If a patient is evaluated for fibroid disease and potential uterine artery embolization, a dynamic oblique coronal magnetic resonance angiography sequence augments the normal precontrast and postcontrast axial imaging, which aids in prediction of anomalous ovarian arterial supply to uterine fibroids.
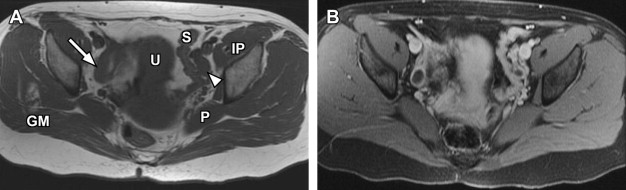
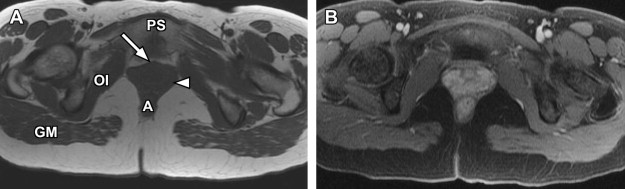
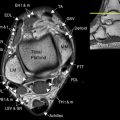
For specific evaluation of the female urethra, a high-resolution protocol is employed. Most patients are examined using an endoluminal coil placed in the vagina to improve signal-to-noise ratio at higher spatial resolutions. The protocol begins with axial, sagittal, and coronal T2-weighted fast spin-echo imaging sequences using a focused small FOV ( Fig. 7 A, C). Subsequently, precontrast axial T1-weighted spin-echo imaging is performed with a similar small FOV. Chemical fat suppression is then added to this T1-weighted sequence, which is repeated for both precontrast and postcontrast imaging (see Fig. 7 B). In patients who are unable to tolerate local coil placement in the vagina, the female pelvis protocol with phased-array surface coil can be substituted, focusing mostly on the perineum rather than the uterus; in this setting, long-axis and short-axis T2-weighted imaging is converted to traditional axial and coronal planes.

Anatomic Considerations and MRI Features
Magnetic resonance has a significant role in evaluation of the pelvic abnormalities, including uterine, ovarian, cervical, adnexal, and congenital abnormalities. A better understanding of anatomy remains crucial in the evaluation of congenital abnormalities as well as in characterization of a lesion and its extent.
Uterus and cervix
The uterus can be divided into 3 segments: fundus, body, and cervix. The uterine body or corpus consists of 3 layers; from central to peripheral, these include the endometrium, junctional zone, and myometrium.
The endometrium is the central most portion of the uterus, with a varying thickness that changes during the menstrual cycle—it is wider during the secretory phase than during follicular phase or menstruation. The normal endometrial thickness varies depending on age, with thickness of less than 10 mm considered normal in reproductive age women, whereas a thickness of less than 5 mm is considered normal in postmenopausal women. Women on hormonal replacement therapy can have endometrial thickness ranging from 5 to 8 mm. The endometrium is typically high signal on T2-weighted images (see Figs. 1–3 ), although not as high signal as simple fluid in the adjacent urinary bladder and homogeneous low signal intensity on precontrast T1-weighted images (see Figs. 4–7 ).
The junctional zone represents the innermost layer of the myometrium and demonstrates a characteristic low signal intensity relative to myometrium on T2-weighted images (see Figs. 1–3 ), likely from a multifactorial basis. These described factors include the presence of compact smooth muscles, decreased water content of the cells versus the outer myometrium, and increased number and size of nuclei compared with outer myometrium. The normal junctional zone can vary in size, but a thickness greater than 12 mm is considered abnormal. The junctional zone can be poorly demarcated in postmenopausal women.
The outer myometrium is structurally different than the junctional zone, with increased cellular free water and decreased cell packing/density. This results in intermediate increased signal intensity on T2-weighted images (see Figs. 1–3 ), substantially higher than the junctional zone but generally lower than the hyperintense endometrium. The entire uterus is of intermediate signal intensity on T1-weighted images relative to muscle with no clear demarcation of different zones (see Figs. 4–7 ).
The cervix is separated from the uterine body by the internal os. Three discrete cervical zones can be identified on high-resolution T2-weighted images. The central hyperintense zone is formed by the endocervical canal with its mucosa, secretions, and plica palmatae/longitudinal folds. Peripheral to this, there is a middle layer, which represents the inner fibromuscular stroma, characterized by hypointense features on T2-weighted imaging due to fibrous stroma and dense smooth muscle. The peripheral outer layer demonstrates a more modest signal intensity, often low-intermediate signal on T2-weighted images, corresponding to the outer fibromuscular stroma. The inner fibromuscular stroma/middle layer is frequently contiguous with the junctional zone in many women, whereas the outermost layer may be contiguous with the outer uterine myometrium. On T1-weighted images, there is no apparent distinction between different layers of the cervix; however, after the administration of intravenous gadolinium contrast, the endocervical mucosa enhances more rapidly than the fibromuscular stroma.
Congenital uterine anomalies comprise a wide spectrum of variant anatomy. The true prevalence of uterine anomalies has been difficult to assess, with varying reported rates between 0.16% and 10%, often confounded by selection bias or variance in classification schemes. Abnormal uterine configurations are typically related to developmental problems of the paramesonephric or müllerian ducts in the first trimester fetus. The paired müllerian ducts join together to form the uterus and upper vagina. After fusion, a midline septum is resorbed as the uterus assumes its normal configuration. Conceptually, müllerian fusion abnormalities can be broadly characterized into 3 categories: agenesis/hypoplasia, defects in vertical fusion, and defects in lateral fusion. The modern and widely used classification scheme developed by the American Fertility Society divides congenital anomalies into 7 discrete classes; associated vaginal or cervical abnormalities are reported as subsets of the major classes. Although many cases can be placed into a single category, it is possible for individual cases to overlap classes or fall along a spectrum of classical disease.
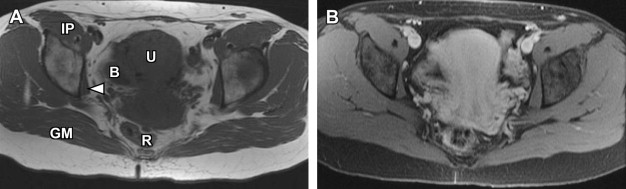
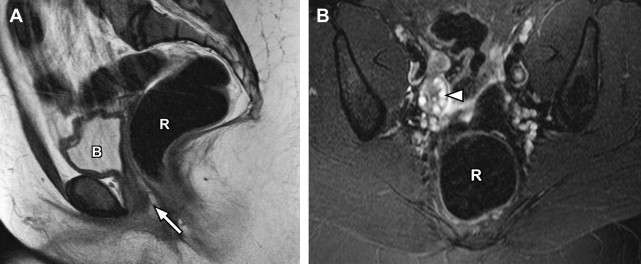
Disorders of agenesis and hypoplasia form the basis for class I anomalies, usually resulting from failure of müllerian duct development before potential fusion. Class I anomalies account for approximately 5% to 10% of all müllerian fusion anomalies. Classically, complete bilateral agenesis of the entire uterus and upper vagina is termed, Mayer-Rokitansky-Küster-Hauser syndrome ( Fig. 8 ). In 10% of such cases, isolated vaginal agenesis may be present with a rudimentary or obstructed uterine remnant in place. Clinically, these patients present with normal female phenotype but demonstrate primary amenorrhea. MRI demonstrates normal female gonads/ovaries but agenesis of the uterus and upper vagina (see Fig. 8 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree