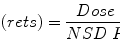, Foster D. Lasley2, Indra J. Das2, Marc S. Mendonca2 and Joseph R. Dynlacht2
(1)
Department of Radiation Oncology, CHRISTUS St. Patrick Regional Cancer Center, Lake Charles, LA, USA
(2)
Department of Radiation Oncology, Indiana University School of Medicine, Indianapolis, IN, USA
Types of Normal Tissue Effects
Early effects occur within 60 days of irradiation and are due to acute cell killing.
These generally involve tissues with rapid cell turnover.
As long as enough stem cells survive to repopulate the tissue, early effects can be completely repaired over time.
Late effects occur >60 days later and are due to effects other than acute cell killing.
Mechanisms include vascular damage, fibrosis, and damage to parenchymal cells in organs.
These generally involve tissues without rapid cell turnover, and cannot be completely repaired.
Consequential Late effects are permanent tissue damage secondary to early effects.
These are caused by a very severe early effect that never completely heals.
For example, skin necrosis requiring a skin graft.
Fraction Size and Treatment Time Effects
Acute effects are less sensitive to fraction size (high α/β) but more sensitive to overall treatment time.
Toxicity based on Total Gy and Gy per week.
Prolonging the course of radiation allows for repopulation.
Late effects are more sensitive to fraction size (low α/β) but less sensitive to overall treatment time.
Toxicity based on Total Gy and Gy per fraction.
Fractionation allows for increased repair, but little or no repopulation occurs within a clinically relevant time span.
When re-treating a previously irradiated area keep in mind:
Late effects are never completely repaired. This is the concept of remembered dose, which decreases the tissue’s tolerance to re-irradiation.
Acute reacting tissues do not “remember” much dose. They can be considered “completely” repaired after 2 months or so, unless severe enough to become consequential late effects.
Stem Cells: Latency and Functional Subunits
In a tissue with dividing stem cells and non-dividing functional cells, the functional cells are largely unharmed by radiation while the stem cells are killed.
There is a latency period before any tissue dysfunction becomes apparent.
The latency time is equal to the lifespan of the functional cell.
Following this, any surviving stem cells will attempt to regenerate the tissue.
Each stem cell can only regenerate a finite volume of organ. This volume is called the functional subunit (FSU).
A structurally defined FSU is an anatomic structure that defines a group of cells.
Kidneys, lungs, livers, and exocrine glands are organized into structurally defined FSUs (nephrons, bronchial trees, portal triads, etc).
A structurally undefined FSU is not an anatomic structure at all.
For example, stem cells in the skin can only migrate a finite distance, but this is not limited by any specific anatomic boundary.
A tissue rescue unit is the minimum number of FSUs required for organ function.
Serial and Parallel Organs and Volume Effect
In a serial organ (CNS, GI tract) loss of function in one part of the organ will cause the entire organ to stop functioning.
Therefore there is no threshold volume – high dose to even a small volume can cause critical injury.
The probability of damage is proportional to the volume irradiated:
If 50 Gy to spinal cord has a 1 %/cm chance of myelopathy, irradiating 1 cm of cord may be reasonable but irradiating 30cm of cord would not be reasonable.
The risk of injury is dominated by the highest dose.
The spinal cord can tolerate 36 Gy to the whole organ, but cannot tolerate 74 Gy to one spot.
In a parallel organ (kidney, lung, liver) loss of function in one part of the organ only affects that part of the organ.
There is a threshold volume effect: you can take out an entire kidney without causing renal failure if the other kidney is healthy.
Partial organ effect does not always correlate with whole organ function.
For example, chest CTs after chest wall irradiation will show changes in a small slice of lung within the radiation field.
However, the rate of symptomatic pneumonitis is very low.
The risk of injury is dominated by the average dose over the whole organ volume.
The lung cannot tolerate 36 Gy to the whole organ, but can easily tolerate 74 Gy to one spot.
Skin and mucosa are neither serial nor parallel but they behave clinically like parallel organs.
This is because desquamating a small area of skin is much more tolerable than desquamating a large area.
Casarett’s Classification of Radiation Sensitivity
Arranged from most sensitive (Group I) to least sensitive (Group IV).
Group I: Vegetative Intermitotic
Divides constantly with no differentiation.
Includes basal epithelial cells (skin, intestinal crypts, etc), undifferentiated hematopoietic stem cells and germ cells.
Group II: Differentiating Intermitotic
Divides for a finite amount of cycles before differentiating into a non-dividing cell.
Includes all of the cells that are intermediate between stem cells and differentiated cells. For example, myelocytes, spermatogonia, etc.
Group III: Reverting Postmitotic
A normally non-dividing cell that retains the potential to divide (“revert”).
Includes liver, kidneys and glandular tissues such as pancreas, adrenal, thyroid and pituitary.
Group IV: Fixed Postmitotic
A permanently non-dividing cell.
Includes permanent cells such as nerves and muscles, as well as short-lived differentiated cells such as neutrophils, red blood cells, and superficial epithelial cells.
Exceptions:
Connective tissue stroma (fibroblasts and endothelium) are intermediate between II and III.
Peripheral blood lymphocytes are incredibly sensitive to apoptosis and are very radiosensitive despite being Group IV.
Other peripheral blood cells (granulocytes, RBCs and platelets) are very radioresistant.
Therefore the latency time of cytopenias after RT is based on the lifespan of the differentiated cell (Fig. 26.1).
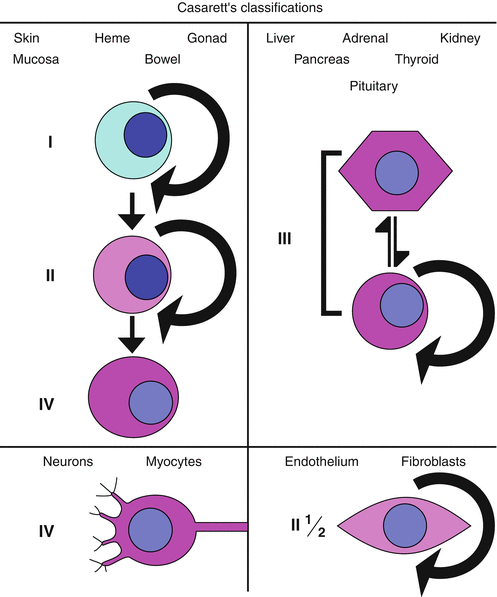
Fig. 26.1
Casarett’s classifications. For skin, mucosa, gonads, bowel and hematologic cells, the progression is from vegetative intermitotic to differentiating intermitotic to fixed postmitotic. For cells of the liver, adrenal gland, kidney, pancreas, thyroid and pituitary, the cells are often reverting intermitotic. Nerve and muscle cells are typically fixed postmitotic by the time of birth (with few exceptions), and endothelium and fibroblasts contain elements that would be described as both reverting intermitotic and differentiating intermitotic.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree