Obstetric Ultrasound
William E. Brant
Imaging Methods. US remains the imaging method of choice for dating the pregnancy, monitoring fetal growth, assessing fetal well-being, and evaluating fetal anatomy and maternal pelvic organs. Transvaginal US is particularly useful in the assessment of first-trimester pregnancy and in the demonstration of fetal anatomic structures deep in the pelvis. Modern US offers superb anatomic detail in real time, keeping up with the frequently vigorous motion of the fetus (1,2). Three-dimensional (3D) volume US provides additional diagnostic information for various conditions including facial anomalies, neural tube defects, and cardiac and skeletal malformations (3). Examination time for standard fetal anatomic surveys may be significantly shortened by use of 3D volume sonography (4). MR is used with increasing frequency as a supplement to US imaging when the US examination is equivocal or when additional anatomic information is needed for appropriate treatment (5). MR offers excellent detail of maternal pelvic organs, unobscured by bone, gas, or fat. Demonstration of fetal anatomy is limited by fetal motion but may be overcome by fetal sedation and fast scan techniques.
Standards for the performance of obstetric US exami-nations have been published by the American Institute of Ultrasound in Medicine (AIUM) and endorsed by the American College of Radiology (ACR) and the American College of Obstetricians and Gynecologists (ACOG) (6). In the first trimester, the location and appearance of the gestational sac is documented. The presence or absence of a yolk sac and embryo is confirmed. If an embryo is present, the crown-rump length (CRL) is measured and fetal cardiac activity is documented. Fetal number is determined and the uterus and adnexa are thoroughly examined. Whenever possible, the fetal neck region should be examined and nuchal translucency (NT) measured. The guidelines for the second and the third trimesters define a standard examination to include fetal presentation, amniotic fluid volume, cardiac activity, placental position, fetal measurements (biometry), fetal number, fetal anatomic survey, maternal cervix, and adnexa. A standard fetal anatomic survey includes the head, face, neck, upper lip, cerebellum, choroid plexus, cisterna magna, lateral cerebral ventricles, midline falx, cavum septum pellucidi, four-chamber heart, outflow tracts, stomach, kidneys, bladder, umbilical cord insertion site, umbilical cord vessel number, the entire spine, and presence or absence of the arms or legs. Fetal gender is determined when medically indicated. A limited examination is performed to answer a specific question such as to verify fetal position or to confirm fetal cardiac activity. Limited examinations are performed generally only when a prior complete examination is on record. When a fetal anomaly is suspected, a specialized examination is performed. Specialized examinations may include fetal echocardiography, biophysical profile, or fetal Doppler sonography.
Use of Doppler in Pregnancy. Evaluation of fetal and maternal circulation by color flow and spectral Doppler adds significantly to obstetric diagnosis (7). However, because all forms of Doppler involve the use of significantly higher levels of acoustic energy than conventional B-mode imaging, these modalities should be used with caution. Energy output from Doppler can be 10 to 15 times more intense than that of B-mode US. When modern US equipment is used at maximum power settings for Doppler examinations, acoustic outputs are sufficient to produce obvious biologic effects, including tissue heating, cavitation, and tissue disruption. Potential cavitation and tissue disruptive effects are most significant in the first trimester when embryologic tissues are tiny and loosely tethered. Thermal effects are more significant in the second and the third trimesters when bone is present increasing sound absorption and heating (7). The International Perinatal Doppler Society and other organizations issue cautions and guidelines for use of Doppler in pregnancy (8,9). US exposures should be as low as reasonably achievable (ALARA), limiting output control and reducing the amount of time that the beam is focused on one site. Doppler US should be used only when the potential medical benefit outweighs any potential risk (7). Obstetric US should not be used for non-medical reasons such as non-medical photos or videos. When imaging the normal embryo in the first trimester, all forms of Doppler should be avoided. In particular, Doppler should not be used to document normal embryonic cardiac activity. M-mode US or recording of real-time US by cine loop provide the same documentation at much lower energies.
First Trimester
The first trimester covers the period from conception to the end of the 13th menstrual week. This includes the entire embryonic period (0 to 10 weeks) and is a time of dynamic
growth and the differentiation and development of most organ systems (1). The embryo and the fetus have the greatest risk of maldevelopment, injury, and death during this period because of external factors (infection, drugs, radiation, etc.) or chromosome abnormalities. About 40% of implanted zygotes are menstrually aborted, and another 25% to 35% of surviving embryos will threaten to abort during the first trimester.
growth and the differentiation and development of most organ systems (1). The embryo and the fetus have the greatest risk of maldevelopment, injury, and death during this period because of external factors (infection, drugs, radiation, etc.) or chromosome abnormalities. About 40% of implanted zygotes are menstrually aborted, and another 25% to 35% of surviving embryos will threaten to abort during the first trimester.
Normal Gestation
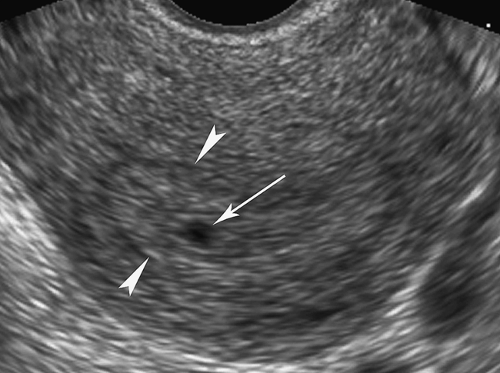
The presence of a pregnancy is confirmed by a positive serum β-human chorionic gonadotropin (β-hCG) test or by a positive enzyme-linked immunosorbent assay (ELISA) urinary pregnancy test. Radioimmunoassay for serum β-hCG allows pregnancy to be detected within 2 weeks of conception (as early as 23 menstrual days) and before a normal gestational sac can be detected by either transabdominal or transvaginal US. The early gestational sac can be seen by transvaginal sonography at 3.5 to 4.5 menstrual weeks as a tiny cystic structure implanted within the echogenic decidua, the intradecidual sign (Fig. 37.1). This sign is not specific for early intrauterine pregnancy and may be mimicked by intrauterine fluid collections or decidual cysts in the presence of ectopic pregnancy. A normal gestational sac is visualized by the transabdominal approach by 5 menstrual weeks. The normal gestational sac appears on US as a smoothly contoured, round, or oval, fluid-containing structure positioned within the endometrium near the fundus of the uterus (Table 37.1). The normal sac has an echogenic border greater than 2 mm thick, which represents the choriodecidual reaction. A double decidual sac sign is evident in about 85% of normal pregnancies. The double sac sign is produced by visualization of three layers of decidua early in pregnancy (Fig. 37.2). The term decidua refers to the endometrium of the pregnant uterus. Hormones of pregnancy, progesterone and others, act on the endometrium to enlarge stromal cells and increase vascularity to promote implantation and development of the gestation. The decidua vera lines the endometrial cavity, and the decidua capsularis covers the gestational sac. The decidua basalis contributes to the formation of the placenta at the site of implantation. A small amount of fluid in the endometrial cavity separates the decidua vera from the decidua capsularis, enabling
visualization of the “double sac.” The free margin of the gestational sac consists of chorion and decidua capsularis and is normally at least 2 mm thick. The double sac is not complete because of placental attachment to the uterine wall. A well-visualized double sac is excellent evidence of intrauterine pregnancy. An absent double sac sign is evidence of an abnormal intrauterine pregnancy or an ectopic pregnancy.
visualization of the “double sac.” The free margin of the gestational sac consists of chorion and decidua capsularis and is normally at least 2 mm thick. The double sac is not complete because of placental attachment to the uterine wall. A well-visualized double sac is excellent evidence of intrauterine pregnancy. An absent double sac sign is evidence of an abnormal intrauterine pregnancy or an ectopic pregnancy.
Table 37.1 Us Characteristics of A Normal Gestational Saca | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
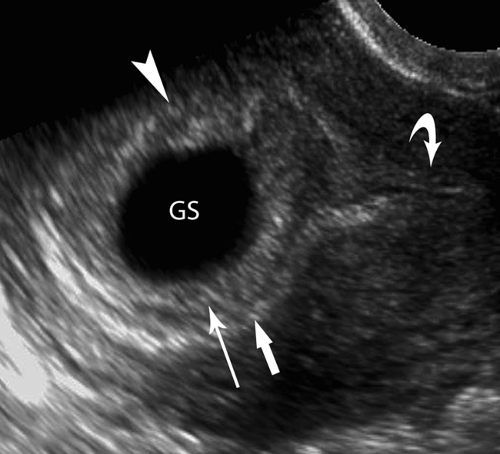
The yolk sac is a 2- to 6-mm diameter, spherical, cystic structure (Fig. 37.3) that is connected to the midgut of the embryo by a thin stalk, the vitelline duct. A Meckel diverticulum is a remnant of the connection of the vitelline duct (also called the omphalomesenteric duct) to the distal ileum. The yolk sac is the earliest site of blood cell formation in the embryo. It floats freely in fluid between the amniotic and the chorionic membranes. It is generally the earliest structure visualized within the gestational sac and serves as definitive evidence of early pregnancy. The yolk sac should always be visualized in normal pregnancy in gestational sacs of 20-mm mean sac diameter (MSD) by transabdominal US or 8-mm MSD by transvaginal US. The yolk sac is generally seen between 5 and 12 weeks gestational age (GA).
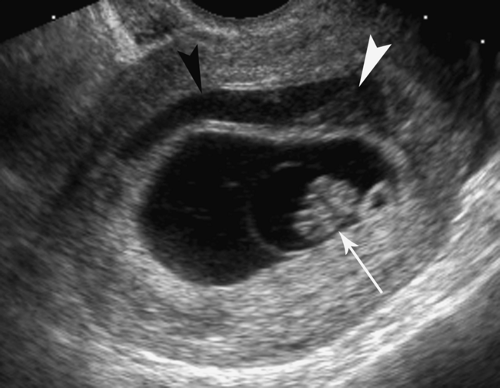
The earliest demonstration of the embryo is the double bleb sign, produced by the amniotic sac and the yolk sac with the embryonic disc between them (Fig. 37.4). Embryos as small as 2 mm can be detected by transvaginal US. The earliest embryonic cardiac activity can be detected by careful inspection of the embryonic disc by real-time US. Transvaginal sonography may demonstrate tiny normal embryos (<5 mm) in which cardiac activity cannot be confirmed. Cardiac activity should always be seen transvaginally in embryos that can be visualized by transabdominal US.
The corpus luteum develops on the ovary at the site of the dominant follicle from which ovulation occurred. The corpus luteum secretes estrogens, progesterone, and other hormones that are essential for establishing and maintaining pregnancy. The wide range of normal appearance of the corpus luteum on US must be recognized for accurate diagnoses of abnormalities of the first trimester (Fig. 37.5) (10). Immediately following ovulation, the corpus luteum appears as an area of focal hemorrhage on the ovary. It soon develops into a cystic structure averaging 2 to 5 cm in size and containing clear fluid or fluid with floating internal echoes or clots of hemorrhage. Color Doppler shows an intense vascularized ring surrounding the corpus luteum and supplying blood flow to support its production of hormones. Acute hemorrhage into the corpus luteal cyst may be a cause of pelvic pain in the first trimester. While a corpus luteum may resemble an ectopic pregnancy, remember that most ectopic pregnancies occur in the tube while the corpus luteum is an ovarian structure. In up to one-third of cases, the corpus luteum may be seen on the ovary opposite to the side of an ectopic pregnancy in the tube.
Normal developmental anatomy of the embryo in the first trimester includes cystic appearance of the rhombencephalon and herniation of the gut into the base of the umbilical cord. Between 6 and 8 weeks GA, the hindbrain (rhombencephalon) forms a prominent cystic structure (Fig. 37.6) that becomes the normal fourth ventricle. Between 9 and 11 weeks GA,
the midgut herniates into the base of the umbilicus forming a physiologic omphalocele seen as a protruding midline anterior abdominal wall mass 6 to 9 mm in size (Fig. 37.7). These normal developmental landmarks must not be mistaken for anomalies.
the midgut herniates into the base of the umbilicus forming a physiologic omphalocele seen as a protruding midline anterior abdominal wall mass 6 to 9 mm in size (Fig. 37.7). These normal developmental landmarks must not be mistaken for anomalies.
Abnormal Pregnancy
Patients who present with vaginal bleeding and pelvic pain during the first trimester are commonly referred for US examination. The differential diagnosis is listed in Table 37.2.
Abortion is the termination of pregnancy before 20 weeks GA. Spontaneous abortion is the termination of pregnancy by natural causes. Approximately 10% to 15% of all known pregnancies end in spontaneous abortion. Up to 60% of spontaneous abortions have chromosomal abnormalities. A number of clinical terms are used to describe abortion. Threatened abortion refers to the occurrence of vaginal bleeding and uterine cramping with a closed cervical os in early pregnancy. Threatened abortion complicates roughly 25% of all pregnancies.
Inevitable abortion presents with cervical dilation and fetal or placental tissues within the cervical os. With complete abortion, all uterine contents have been expelled. Incomplete abortion refers to the presence of residual products of conception within the uterus. In a missed abortion, the fetus has died but remains within the uterus. Habitual abortion is defined as three or more successive spontaneous abortions. Anembryonic pregnancy or blighted ovum is a pregnancy in which the embryo has died and is no longer visible or never developed.
Inevitable abortion presents with cervical dilation and fetal or placental tissues within the cervical os. With complete abortion, all uterine contents have been expelled. Incomplete abortion refers to the presence of residual products of conception within the uterus. In a missed abortion, the fetus has died but remains within the uterus. Habitual abortion is defined as three or more successive spontaneous abortions. Anembryonic pregnancy or blighted ovum is a pregnancy in which the embryo has died and is no longer visible or never developed.
“Empty” Gestational Sac. A gestational sac without an embryo demonstrated by US may be a very early intrauterine pregnancy or a nonviable intrauterine pregnancy (anembryonic pregnancy) (Fig. 37.8). An empty gestational sac must be differentiated from a pseudogestational sac associated with ectopic pregnancy (see Fig. 37.10). A gestational sac is considered to be abnormal if it demonstrates the following features (Table 37.3): large size without an embryo or yolk sac, distorted shape, irregular contour, thin or weak choriodecidual reaction, absence of a double decidual sac, or abnormal position. Any one of the major criteria or three of the minor criteria are considered diagnostic of a failed pregnancy. Large sac size without visualized yolk sac or embryo and a distorted sac contour have a reported 100% specificity and positive predictive value for identification of nonviable pregnancy. Most authors recommend allowing a 1- to 2-mm margin of error and repeating any equivocal scans in several days. Growth of the gestational sac of less than 1 mm/d MSD is strong evidence of abnormal sac development.
Embryonic or fetal demise is diagnosed by US confirmation of the absence of cardiac activity. Absence of cardiac activity in a fetus or an embryo large enough to be visualized by transabdominal US is definitive evidence of death. Because of the increased sensitivity of transvaginal US in demonstrating cardiac activity, all cases of suspected demise of small embryos should be confirmed by transvaginal US, which may demonstrate cardiac activity even in embryos as small as 1.5 mm CRL. Transvaginal US may also visualize small, normal, living embryos (<5 mm CRL) without demonstrating cardiac
activity. Absence of cardiac activity in embryos larger than 5 mm on transvaginal US is considered diagnostic of embryonic demise (missed abortion). Embryos smaller than 5 mm without cardiac activity should be rescanned in a few days to confirm viability. M-mode US is used to document visualized cardiac activity.
activity. Absence of cardiac activity in embryos larger than 5 mm on transvaginal US is considered diagnostic of embryonic demise (missed abortion). Embryos smaller than 5 mm without cardiac activity should be rescanned in a few days to confirm viability. M-mode US is used to document visualized cardiac activity.
Table 37.2 Vaginal Bleeding in the First Trimester Differential Diagnosis | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Table 37.3 Ultrasound Characteristic of an Abnormal Gestational Saca | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Quantitative serum β-hCG levels have been correlated with US findings to assist in the identification of abnormal pregnancies. Great caution is advised, however, because of differences in the way that individual laboratories report serum β-hCG results and in the wide variation in values obtained by different laboratories. Discriminatory levels will vary with the resolution of the US scanner used and with the laboratory reporting the hormonal assay. Serial measurements are the most useful. A normal intrauterine pregnancy demonstrates serum β-hCG levels that double every 48 hours. A failure of doubling indicates an abnormal pregnancy.
Ectopic pregnancy occurs in only 2% of all pregnancies, but it is the major cause of pregnancy-related maternal deaths (11,12,13). Misdiagnosis of ectopic pregnancy remains one of the most common areas for medical malpractice litigation. Patients at high risk for ectopic pregnancy include those with a history of pelvic inflammatory disease, tubal surgery, endometriosis, ovulation induction, previous ectopic pregnancy, or use of intrauterine device (IUD) for contraception. Most ectopic pregnancies (95%) occur in the fallopian tube, most commonly in the isthmic portion. Interstitial ectopic pregnancies (2% to 5%), developing in the portion of the tube passing through the uterine wall may grow to large size before rupture, resulting in catastrophic hemorrhage. Rare sites for ectopic implantation include the abdominal cavity, ovary, and cervix. All patients with a positive pregnancy test (serum β-hCG), vaginal bleeding, pelvic pain, or adnexal mass must be considered at risk for ectopic pregnancy.
A completely confident diagnosis of ectopic pregnancy can be made sonographically only when a living embryo or a gestational sac containing a yolk sac is positively demonstrated to be in a position outside of the uterus (18% to 26% of ectopic pregnancies). Transvaginal US increases the possibility of demonstrating a live ectopic pregnancy. In any other circumstance, we are dealing with a situation of relative risk (Table 37.4). When an intrauterine pregnancy is documented by US, the risk of coexisting ectopic pregnancy is extremely low, estimated at 1 in 30,000. Concurrent intrauterine and extrauterine pregnancies (termed heterotopic pregnancy) do occur, however, especially in patients taking ovulation-inducing drugs, in whom the risk of ectopic pregnancy is increased to 1 in 6000 to 7000. The risk of ectopic pregnancy is high when the uterus is empty (no gestational sac) and an adnexal mass, other than a corpus luteal cyst, is demonstrated. Similarly, an ectopic pregnancy is likely when the uterus is empty and a moderate or large amount of echogenic fluid or blood clots are seen in the cul-de-sac. Even when the US examination is entirely normal but without definitive evidence of intrauterine pregnancy, a patient with a positive pregnancy test remains at risk for ectopic pregnancy. The role of US, then, is to demonstrate findings that determine relative risk. This assessment, in conjunction with clinical history and physical examination, determines the risk of ectopic pregnancy and the next step in the patient’s evaluation.
Table 37.4 Risk of Ectopic Pregnancy As Determined By Ultrasound Findings | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
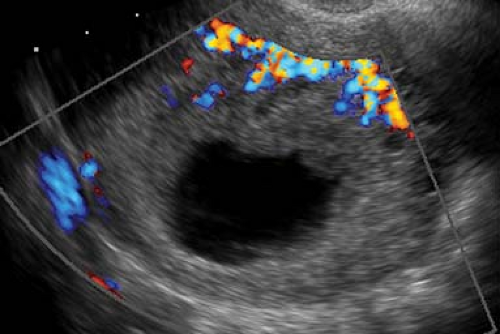
US findings in ectopic pregnancy include demonstration of an extrauterine gestational sac appearing as a fluid-containing structure with an echogenic ring, the tubal ring sign (40% to 68% of ectopic pregnancies) (Fig. 37.9). A living or dead embryo might or might not be evident. The ectopic gestational sac must be differentiated from a corpus luteal cyst, which develops on the ovary at the site of ovulation. Clotted blood from hemorrhage within a corpus luteal cyst may simulate an embryo. A key differentiating finding is whether the cystic mass arises from the ovary. Most ectopic pregnancies occur within the fallopian tube and can be shown on real-time transvaginal US to be separate from the ovary. Implantation of ectopic pregnancy on the ovary is a rare event. Corpus luteal cysts always arise from the ovary. Hematosalpinx or ruptured ectopic pregnancy may appear as an amorphous solid or complex adnexal mass (a hematoma) lacking an embryo or sac. Blood in the cul-de-sac usually appears as echogenic fluid but may be entirely echolucent if liquid or echogenic and solid appearing if clotted. Moderate or large volumes of echogenic fluid or blood clots in the cul-de-sac are highly predictive of ectopic pregnancy. A small volume of anechoic fluid in the cul-de-sac is a common and normal finding.
In the presence of an ectopic pregnancy, the lining of the uterus will be thickened and echogenic reflecting the conversion of endometrium to decidua induced by the hormones of pregnancy. Blood in the uterine cavity produces cystic appearing mass termed a pseudogestational sac in 10% to 20% of ectopic pregnancies (Fig. 37.10). A true gestational sac is differentiated from “pseudosac” by the presence of a yolk sac or embryo. A double decidual sac sign suggests a true gestational sac, but is not totally reliable because some pseudosacs may also show a double decidual sac sign. Pseudosacs are located centrally within the uterine canal whereas a normal true gestational sac is eccentrically implanted within the
decidua. Doppler studies demonstrate absent or minimal peritrophoblastic flow with pseudosacs and high-velocity, low-impedance flow with true gestational sacs.
decidua. Doppler studies demonstrate absent or minimal peritrophoblastic flow with pseudosacs and high-velocity, low-impedance flow with true gestational sacs.
 Figure 37.9. Ectopic Pregnancy. A. Transvaginal US in a longitudinal plane demonstrates an empty uterus (between calipers, +, x) in a pregnant patient. Echogenic blood (arrow) distends the cul-de-sac. B. Transverse transvaginal image reveals a tubal ring sign (arrow) in the right adnexa highly indicative of ectopic pregnancy. U, uterus (between calipers, +). C. Color Doppler image of a tubal ectopic pregnancy shows a tubal ring sign with a “ring of fire” made up of prominent blood vessels. Note the similarity in appearance to the corpus luteal cyst in Figure 37.5A. Differential is made by real-time US determination of the location of the mass as arising from the ovary or as being separate from the ovary. This differentiation is not always possible. |
Definitive therapy for ectopic pregnancy is surgical resection of the involved fallopian tube. Medical management using methotrexate as a cell-growth inhibitor has become increasingly popular because tubal patency can be preserved.
Subchorionic hemorrhage is a common finding in the bleeding pregnant patient before 20 weeks GA (14). All cases are believed to develop because of venous bleeding from separation of the margin of the placenta. The hematoma collects preferentially beneath the chorion because the chorion is more easily separated from the myometrium, than is, the placenta. Patients may be asymptomatic if the hematoma remains confined or may present with vaginal bleeding if the hematoma leaks through the cervix. In most patients, a subchorionic hematoma is an innocent finding; however, an increased rate of spontaneous abortion has been reported in some series associated with large hematomas (>60 mL), advanced maternal age (>35 years), and early GA (<8 weeks). The US appearance of the hemorrhage varies with age (Fig. 37.11). Acute bleeding is anechoic to hypoechoic. With clotting, it becomes hyperechoic and heterogeneous. With lysis, the hematoma reverts to being hypoechoic to anechoic.
Implantation bleeding is a nonspecific term that refers to small collections of blood at the site of attachment of the chorion to the endometrium. These are in essence small areas of subchorionic hemorrhage that occur early in pregnancy. US follow-up is warranted to assess for progression.
Retained Products of Conception (RPOC). Following a spontaneous or induced abortion, or even a normal delivery, the patient may continue to have vaginal bleeding caused by incomplete expulsion of the pregnancy. The most common US
appearance of RPOC is an echogenic mass within the uterine cavity representing retention of a portion of the placenta (Fig. 37.12) (15). The mass, like the normal placenta, is more echogenic than the myometrium. A polypoid or pedunculated mass of retained placental tissues has been termed a placental polyp. Blood clots appear as hypoechoic masses without blood flow within the uterine cavity. Variants in appearance of RPOC include irregular thickening of the endometrium (>10 mm), highly reflective structures with shadowing representing fetal parts or calcified placental remnants or mixed echogenicity masses representing necrotic tissue. Color Doppler findings are highly variable showing little or no vascularity within devascularized RPOC or striking blood flow within the mass (Fig. 37.12B) and the myometrium resembling a uterine arteriovenous malformation (16).
appearance of RPOC is an echogenic mass within the uterine cavity representing retention of a portion of the placenta (Fig. 37.12) (15). The mass, like the normal placenta, is more echogenic than the myometrium. A polypoid or pedunculated mass of retained placental tissues has been termed a placental polyp. Blood clots appear as hypoechoic masses without blood flow within the uterine cavity. Variants in appearance of RPOC include irregular thickening of the endometrium (>10 mm), highly reflective structures with shadowing representing fetal parts or calcified placental remnants or mixed echogenicity masses representing necrotic tissue. Color Doppler findings are highly variable showing little or no vascularity within devascularized RPOC or striking blood flow within the mass (Fig. 37.12B) and the myometrium resembling a uterine arteriovenous malformation (16).
Gestational Trophoblastic Disease
Gestational trophoblastic disease is a group of neoplasms that range from benign to highly malignant (14,17). All are derived from abnormal placental tissues and occur as sequelae to pregnancy. Both benign and malignant tumors produce β-hCG. Marked elevation of serum β-hCG levels is characteristic, and serial measurement is a sensitive and reliable indicator of tumor activity. Gestational trophoblastic disease complicates about one in 1000 to 2000 pregnancies in the United States but has a much higher incidence in the Far East and in Latin America (17). Women over age 40 and those with a prior history of molar pregnancy are also at increased risk.
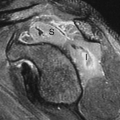
Hydatidiform mole is the most common (80%) and most benign form of the disease but maintains a potential for malignant sequelae. The placenta demonstrates edema and proliferation of trophoblasts. The villi become swollen and vesicular, resembling a bunch of grapes. Patients present with hyperemesis, pregnancy-induced hypertension, and vaginal bleeding. The uterus may be enlarged (50%), normal (35%), or small (15%) for dates. Two types of hydatidiform mole exist. Complete mole (classic mole) (70%) involves the entire placenta, lacks a fetus, and is diploid in karyotype. Partial mole (30%) involves only a portion of the placenta, is associated with an abnormal fetus that is triploid in karyotype (due to fertilization of an ovum by two sperm). This condition is lethal to the fetus. Rarely, a normal fetus may coexist with a complete mole in a twin pregnancy. Prognosis for the normal fetus in these cases is grim because of maternal complications of the mole.
US of complete mole in the first trimester classically shows the uterus to be filled with an echogenic, solid, highly vascular mass, often described as “snowstorm” in appearance (18). The tiny vesicles that make up the mole are too small to be resolved as discrete cysts but cause innumerable sound reflective interfaces that produce the brightly echogenic appearance (Fig. 37.13) (17). As the vesicles enlarge in the second trimester, individual cysts 2 to 30 mm in size become apparent producing a “bunch-of-grapes” appearance. Partial mole demonstrates vesicular changes in only a portion of the placenta. The associated triploid fetus has multiple anomalies.
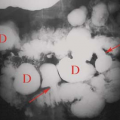
The classic appearance of mole is not always evident. Molar pregnancy may occasionally appear as an anechoic fluid collection that mimics anembryonic pregnancy. Theca lutein cysts are seen as large, septated, bilateral cysts massively enlarging the ovaries in 25% to 65% cases of molar pregnancy (Fig. 37.14). Theca lutein cysts result from hyperstimulation of the ovaries by high circulating levels of β-hCG and are most commonly seen in molar pregnancy in the second trimester.
The classic appearance of mole is not always evident. Molar pregnancy may occasionally appear as an anechoic fluid collection that mimics anembryonic pregnancy. Theca lutein cysts are seen as large, septated, bilateral cysts massively enlarging the ovaries in 25% to 65% cases of molar pregnancy (Fig. 37.14). Theca lutein cysts result from hyperstimulation of the ovaries by high circulating levels of β-hCG and are most commonly seen in molar pregnancy in the second trimester.
Invasive mole (chorioadenoma destruens) refers to invasion of molar tissue into, but usually not beyond, the myometrium. It is seen in about 10% of patients and usually becomes evident after treatment for hydatidiform mole. US shows penetration of the echogenic trophoblastic tissue into the myometrium (18). MR is more sensitive than US in demonstrating the muscle invasive disease seen as focal myometrial masses, dilated vessels, and areas of hemorrhage and necrosis.
Choriocarcinoma is a highly aggressive malignancy that forms only trophoblasts without any villous structure. Choriocarcinoma is locally invasive, spreads into the myometrium and parametrium, and hematogenously metastasizes to any site in the body. Serum β-hCG levels that rise or plateau in the 8 to 10 weeks following evacuation of molar pregnancy suggest invasive or metastatic gestational trophoblastic disease. Choriocarcinoma at any site produces a highly echogenic solid mass.
Fetal Measurements and Growth
Dating the pregnancy and determining the appropriateness of fetal growth are essential to obstetric care. Clinical dating is based on history of the mother’s last menstrual period (LMP) and physical examination assessment of uterine size. Sonographic dating is based on measurements of the gestational sac and the embryo or fetus. Serial measurements of fetal parameters are used to document growth. By convention, pregnancies are dated from the first day of the LMP. The terms gestational age, which is the clinical standard, and menstrual age are usually considered to be synonymous terms and are based on the average 28-day menstrual cycle. Conception is assumed to occur 14 days following the LMP. Term is 40 weeks, with an acceptable range of 37 to 42 weeks.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree