, Melvin D’ Anastasi1 and Wieland Sommer1
(1)
Institute of Clinical Radiology, Ludwig-Maximilians-University Hospital, Munich, Bavaria, Germany
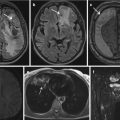
There are major differences how to deal with incidental findings in study participants and patients. While a study participant might see himself as part of a research project, he is less likely to expect diagnoses from cross-sectional imaging examinations. In contrast, a patient in a clinical setting undergoes imaging examinations for a particular reason, that is, to exclude, confirm, or follow up a certain diagnosis. Therefore, the patient expects a particular – positive or negative – report related to the original clinical question. Additional findings not related to the initial indication for the examination are generally reported. These incidental findings need to be handled carefully both by the reporting radiologist and by the physician in charge. Well-considered recommendations given by the radiologist are the most important part of handling incidental findings responsibly. Depending on certain parameters, such as the chosen modality or the image quality, differentiating between “normal” and “pathological” becomes a real challenge for several incidental findings. The reporting radiologist has to decide how to report and assess those incidental findings. By now, there are several recommendations by different societies and committees that can help radiologists in the assessment of incidental findings. In this chapter, we aim to give a brief overview of the most helpful recommendations, which refer to the most frequently occurring incidental findings on thoracic and abdominal CT or MRI.
1 Pulmonary Incidental Findings
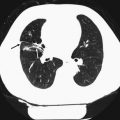
For the assessment of pulmonary nodules, the guidelines of the Fleischner Society are well established. Their recommendations for solid and subsolid lung nodules can help the radiologist in classifying a finding as (most likely) benign and advising follow-up examinations.
1.1 Small Pulmonary Nodules
Small pulmonary nodules are very common findings. They can be detected in scans that involve the whole chest, for example, a trauma scan after a car crash, as well as in scans that only show parts of the lung parenchyma such as a contrast-enhanced CT scan of the supra-aortic arteries. The likelihood increases with the age of the patient collective, and is higher in smokers than in nonsmokers. With current modern scanners, detecting even the smallest nodules with 1–2 mm in diameter has become routine. Since only a slight percentage of incidentally detected, small pulmonary nodules will be malignant, controlling all of them several times is not feasible. Therefore, the Fleischner Society published a position paper in 2005 (MacMahon et al. 2005). This paper should provide practical guidelines for the management of incidentally detected, small pulmonary nodules. The given recommendations apply to adult patients (>35 years) without any known or suspected malignant disease and without fever. The guidelines are based on several follow-up studies evaluating the risk of having or developing lung cancer when a small pulmonary nodule is found. For this assessment, several characteristics of incidental, small pulmonary nodules need to be taken into consideration, such as nodule size, growth rate, and risk factors: the larger the nodule the more likely it is malignant, and follow-ups need to be more frequent. Growth rates of lung nodules differ between ground-glass opacities, ground-glass opacities with a solid component, and solid nodules, with solid nodules showing the shortest mean volume-doubling time. Furthermore, the relative risk for developing lung carcinoma is an important parameter, with smoking being the most important risk factor. For example, the Fleischner Society follow-up and management recommendations for incidentally detected, small pulmonary nodules say that no follow-up is needed for a nodule smaller than 4 mm in a patient with a minimal or absent history of smoking and of other known risk factors. If a nodule of the same size is found in a patient with a history of smoking or with other known risk factors, a follow-up CT after 12 months is recommended. If the nodule size is unchanged, no further scans are required. But, it needs to be considered that a ground-glass or partly solid nodule may require a longer follow-up to exclude indolent adenocarcinoma due to a longer mean volume-doubling time of nonsolid nodules (MacMahon et al. 2005). Equivalent recommendations are given for nodules with a size between 4 and 6 mm, 6 and 8 mm, and for those larger than 8 mm (for further details, please see the table “Recommendations for follow-up and management of nodules smaller than 8 mm detected incidentally at nonscreening CT” (MacMahon et al. 2005)).
1.2 Subsolid Pulmonary Nodules
The recommendations mentioned above already cover a significant proportion of the different types of incidentally detected lung nodules. However, these guidelines lack a detailed consideration of subsolid lung nodules. Therefore, the Fleischner Society provided additional recommendations for the management of subsolid pulmonary nodules in 2012 (Naidich et al. 2013). The term “subsolid” in this paper encompasses the entity of “pure ground-glass nodules” (pure GGN) where no solid component is present and the “part-solid ground-glass nodules” (part-solid GGN) that include a solid component. An important difference between the guidelines from 2005 and the additional recommendations from 2012 is that there is no low-risk/high-risk distinction between smokers and nonsmokers. The main characteristics are the overall size of the lesion(s) and the size of the solid component, if present.
For image acquisition and quality, contiguous thin sections (1 mm) reconstructed with narrow and/or mediastinal windows are recommended to evaluate the solid component. Additionally, wide and/or lung windows will be needed to evaluate the nonsolid component of nodules. The authors further advise the use of a consistent low-dose technique.This is of particular importance in cases for which prolonged follow-up scans are recommended as well as in younger patients. If several scans are available over time, it is important to always compare with the original baseline study to detect subtle changes in growth (Naidich et al. 2013). For example, a solitary, pure GGN ≤5 mm would not require a follow-up CT scan according to the “Recommendations for the management of subsolid pulmonary nodules detected at CT.”. Whereas a solitary, pure GGN >5 mm requires a follow-up at 3 months. If the GGN is unchanged in this scan, an annual surveillance for a minimum of 3 years is recommended. If multiple, pure GGN ≤5 mm are found, a follow-up at 2 and 4 years is recommended, and alternate causes for those multiple nodules should be considered. (For supplementary details, please see the table “Recommendations for the management of subsolid pulmonary nodules detected at CT” (Naidich et al. 2013)).
1.3 Pulmonary Perifissural Nodules
Pulmonary perifissural nodules (PFN) represent another important entity of pulmonary nodules commonly seen on chest scans. It is likely that the majority of these nodules represent lymph nodes. This can be hypothesized by their demonstrated growth rate (they can expand or regress over time), morphological features, and resected PFN. Adequate assessment of pulmonary nodules as PFN plays an important role in reducing the number of recommended follow-up scans. Within the framework of the Dutch–Belgian Randomised Lung Cancer Screening Trial (NELSON), de Hoop et al. have been evaluating PFN over time. Perifissural nodules have been categorized as typical PFN, atypical PFN, and non-PFN. A typical PFN was defined as fissure-attached, homogeneous, solid nodule with smooth margins and a triangular, lentiform, or oval shape. An atypical PFN was not fissure-attached, but perifissural, otherwise showing all features of a typical PFN. All other nodules not meeting these criteria were defined as non-PFN, including spherical or speculated nodules. In the study of de Hoop et al., none of the typical or atypical-defined PFN showed signs of malignancy in the 5.5 years of follow-up (de Hoop et al. 2012).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree