MR sequence
Image resolution
Image contrast
Anatomic coverage
Neurodegenerative focus
T1-3D-MPRAGE
1.0 mm isotropic; sagittal
T1w
Whole brain and upper spinal cord
2D-FLAIR
4.0 mm slice thickness; axial; 0.9 mm voxel size in-plane
T2w
Whole brain
Cardiovascular focus
MRA 3D-SPACE-STIR
2.5 mm slice thickness; coronal; 1.2 mm voxel size in-plane
T2w
Lung apices to diaphragm
Cine SSFP LAX
6.0 mm slice thickness; 1.5 mm voxel size in-plane
SSFP
4-, 3-, 2-chamber view
Cine SSFP SAX
7.0 mm slice thickness; 1.7 mm voxel size in-plane
SSFP
12 short-axis stacks covering base to apex
Thoracoabdominal focus
T2-HASTE
5.0 mm slice thickness; axial; 1.4 mm voxel size in-plane
T2w
Shoulder to epigastric region
T1-3D-VIBE-DIXON
3.0 mm slice thickness; axial; 1.4 mm voxel size in-plane
T1w
Neck to knee
Musculoskeletal focus
PD-FS-3D-SPACE
1.0 mm isotropic; coronal
PD
Pelvis including iliosacral joint and both hips
T2-2D-TSE Spine
3.0 mm slice thickness; sagittal; 1.0 mm voxel size in-plane
T2w
Lumbar, thoracic, cervical spine

Fig. 1
Design of MRI study within the German National Cohort (GNC). While 200,000 subjects will be enrolled across 18 sites in Germany (green areas), about 30,000 subjects will undergo whole-body MR imaging. Thus, five dedicated MR scanners were installed (blue squares). In addition, four imaging cores have been established for central functions, in Munich for coordination and training, in Bremen for data management, in Greifswald for quality assurance, and in Heidelberg for incidental findings (gray squares). The imaging core for incidental findings has developed the basic concept for the management of MR-based incidental findings within the GNC. It provides daily support and advice to the five imaging sites and performs quality control regarding the reporting of incidental findings (Source: The German National Cohort Study)
2 Ethical Framework for IF-Reporting
While most people of the general population could be considered fairly healthy, it is expected that imaging would occasionally lead to the discovery of illnesses of varying degree of medical importance (Lumbreras et al. 2010a, b). Based on the results of similar previous cohort studies, we estimated prospectively that “clinically relevant” IFs can be found in 10 % of the population undergoing MR imaging, considering the targeted age range and morbidity in Germany (Bamberg et al. 2015; Hegenscheid et al. 2013). Therefore, guidance was sought from the ethical commissions of the involved organizations to establish an ethical framework that would help in the management of any finding out of the ordinary, generally designated as IF.
General principles to be considered in the management of IFs were (Radiologists, T.R.C.o and Management of Incidental Findings detected during Research Imaging 2011; Weiner 2014) as follows:
Responsibility for the well-being of the participant: A participant should be informed about health concerning IFs. This is in accordance with European and international ethical guidelines, for example, the Article 26 of the Additional Protocol to the Convention on Human Rights and Biomedicine, concerning Biomedical Research of the Council of Europe (Additional Protocol to the Convention on Human Rights and Biomedicine and concerning Biomedical Research 2007; Convention for the protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine 1999).
Responsibility for the well-being of the society: The general population might be affected from undisclosed illnesses a participant might suffer from. This includes, for example, illnesses that might carry an increased risk for the participant to cause a traffic accident. This is in accordance with the Article 26 of the Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine of the Council of Europe (Convention for the protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine 1999).
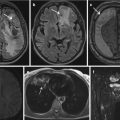
While these general ethical principles seem to be simple and straight forward, implementation presents certain challenges which will likely never be solved satisfactorily. The simple idea of classifying findings into reportable and non-reportable gets confounded by the definition of “IF” itself. While IF might ideally relate to a diagnosis, imaging by itself, even in clinical settings, rarely allows an abnormality to be specified down to a final diagnosis. IFs in MRI exams can present any form of untypical imaging characteristics, for example, a hyperintensity where it is not expected; a broad clinical description such as a cystic lesion; or a likely but not certain diagnosis such as an adrenal gland adenoma. Generally, only an accurate and established diagnosis allows for a reliable estimation of the impact for a participant’s future health.
With ethical principles referring rather to diagnoses but imaging generally providing much less defined information, it becomes apparent that it is often unclear how to classify an IF into report-worthy or not. In clinical as well as in research settings, an innocuous finding wrongly reported as a false-positive illness may cause severe psychologic and bodily harm conflicting with the general bioethical principle of primum non nocere (do not harm). It may also unnecessarily increase health care spending, costs for society, and lead to occupational and insurance-related consequences.
3 Defining Problems in IF-Reporting
Following the ethical considerations set forth by international guidelines (Additional Protocol to the Convention on Human Rights and Biomedicine and concerning Biomedical Research 2007; Convention for the protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine 1999; International Ethical Guidelines for Biomedical Research Involving Human Subjects 2002), a process dubbed “IF-reading” was established. IF-reading is a procedure described by SOPs developed by the researches of the GNC and approved by the ethical commissions of the involved organizations. Those SOPs are to ensure that every participant’s imaging data is assessed by a board-certified radiologist within a certain time frame to detect IFs that might warrant a notification of the participant. Participants are only notified in case of “clinically relevant” IFs. This process poses some intricate difficulties different from IFs encountered during clinical exams. Considerations that need to be accounted for in the particular research setting of the GNC will be presented in the following paragraphs.
3.1 Scientific Imaging Sequences
Imaging sequences in the GNC, as in many other research projects, differ from clinically used sequences. They often do not have the particular purpose to obtain a certain clinical diagnosis. In the GNC, MR sequences were chosen with an emphasis on maximizing morphologic data acquisition in a restricted time frame, sacrificing some of the MRI-inherent benefits of analyzing a lesion based on a multitude of MR-characteristic tissue features. Therefore, IF-reading has to be based mostly on T1- and T2-weighted images without common, clinically applied sequences such as diffusion-weighted imaging (DWI), susceptibility-weighted imaging (SWI), etc. Due to their invasive nature, contrast agents, gut motility suppressing medications, bowel distending procedures, and endorectal/endovaginal coils have also been forgone. With the limited imaging set to characterize a finding, great uncertainty in specifying a finding and a large list of differential diagnoses including artifacts has to be expected.
While reacquisition of only one sequence is allowed, more reacquisitions, for example, because of motion or breathing artifacts, cannot be afforded due to time restrictions. Similarly, sequence protocols are fixed for comparability. No sequences can be swapped for, for example, less motion susceptible ones or more lesion appropriate ones, as it happens in clinical settings. This substantially reduces the sensitivity to pick up a lesion and hugely widens the gap between the ability to detect and characterize a finding.
3.2 Limited Clinical Context
To afford unbiased reporting, and because of strict German privacy and data protection laws, radiologists are blinded to personal and clinical information of the participant, except for gender and the year of birth. Moreover, no data from exams conducted in other areas of the GNC (e.g., blood tests) are shared with the radiologist in charge of the IF-reporting. This severely hampers guidance toward a probable diagnosis of an observed lesion.
3.3 Disproportionate Increase of False Positives
The probability of a lesion being a certain diagnosis is possibly distorted by the fact that examinees randomly selected from the general population are more likely to be healthy individuals, in contrast to patients with clinical indications for imaging. It is a mathematical phenomenon that the positive and negative predictive value of a particular imaging finding depends on the prevalence of the appendant disease in the examined population (Bender et al. 1998). Compared to a clinical setting where MRI is often applied to further characterize an already known or suspected lesion, a mostly healthy general population leads to a lower positive predictive value and accordingly to an increase in false-positive reports. Taking the generally poorer specificity of the applied scientific imaging sequences compared to clinical sequences into account, the report of a potentially harmful finding would come at the expense of an even larger number of false positives. Along with health care costs, physical and psychologic side effects of follow-up procedures would increase disproportionately, compared to true positive disease detection. The effect of false-positive reports is aggravated by the fact that possibly healthy individuals, that otherwise would not have been subjected to medical exams, might undergo harmful or side effect-stricken follow-up investigations.
3.4 Uncertainty Causing Out-of-Proportion Work-Up
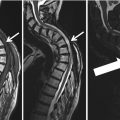
The notification of a participant would likely trigger a clinical work-up outside the GNC. Participants would seek advice from their primary care physicians who would be forced, out of lack of more complete information, to follow up on IFs, likely starting with proper clinical imaging. While this is the intended purpose of the IF-reading to prevent harm from serious illnesses like cancer, for example, this would, under certain circumstances, lead to unnecessary and unnecessarily exaggerated work-up. While this is obvious for false-positive reports, this would also be the case for certain true positive reports, namely, when there is uncertainty about the clinical significance of findings. This includes minor ailments, anatomic variations within the normal range, illnesses that would usually be diagnosed and followed up on a less extensive and costly way, or illnesses that would not receive work-up at all at this point in time. Examples might be an Arteria lusoria occasionally leading to swallowing problems, a hiatal hernia that might or might not be clinically manifest, or the ubiquitous age-related degenerative joint or spine disease that might occasionally explain a participant’s pain but otherwise would not need extensive or no work-up at all.
3.5 Reliability, Reproducibility, and Consistency
For the assessment of 30,000 MR scans acquired at five different sites, a relatively large number of radiologists are involved in reading the acquired data. Furthermore, with this imaging round expected to last at least 4 years, a significant fluctuation of involved radiologists is anticipated. Therefore, high-quality standards must be met to ensure consistency and reliability. It is well known from reproducibility studies that variability is induced by radiologists in image interpretation and diagnoses making (Robinson 1997). Considering the fundamental obligation to provide the same service and the same quality of service to each of the participants, variability should be limited as much as possible. This can be theoretically achieved by either reducing the number of involved radiologists, and/or by involving only highly and specifically trained radiologists, and/or by standardized reporting and/or by conducting quality assurance in IF-reporting.
4 Translating the Ethical Framework into a Reporting Algorithm
Considering the abovementioned restrictions, it became clear that reporting every possible disease would necessitate extensive clinical follow-up with significant over-reporting and disproportionate work-up. Individuals might thus come to harm from non-disclosure of disease states as well as from reporting every possible disease state. Therefore, the ethical framework was defined more precisely in an effort to find the possibly best balance between informing participants about relevant illnesses and avoiding reporting of irrelevant illnesses. To that end a robust system guiding radiologists in IF-reporting was established curtailing especially possibly minor illnesses with questionable relevance, normal variants, and highly uncertain diagnoses.
4.1 The List: An Approach to Define Clinically Relevant IFs
It was decided to develop a specified, categorized, and concise list of reportable findings, limiting uncertainty and false positives as well as establishing consistency. The ground work for this system was laid by expert radiologists familiar with the applied sequences and the ethical considerations.
The ratios of false-positive and false-negative findings are significantly determined by the applied MR sequences. As these ratios are specific to the set of sequences used, comparability with previous cohort studies using different imaging protocols might be severely hampered. Based on the extrapolation of extensive literature research data (excerpt (Abeloos and Lefranc 2011; Al-Shahi Salman 2007; Atalay et al. 2011; Ballantyne 2008; Beigelman-Aubry et al. 2007; Berland et al. 2010; Berlin 2011; Boland et al. 2008; Booth et al. 2012; Borra and Sorensen 2011; Bradley et al. 2011; Childs and Leyendecker 2008; Chow and Drummond 2010; Cordell 2011; Cramer et al. 2011; de Rave and Hussain 2002; Erdogan et al. 2007; Esmaili et al. 2011; Gore et al. 2011; Gross et al. 2010; Hartwigsen et al. 2010; Hoggard et al. 2009; Illes 2008; Irwin et al. 2013; Johnson et al. 2011; Kamath et al. 2009; Khosa et al. 2011; Ladd 2009; Lee et al. 2011; Legmann 2009; Lund-Johansen 2013; MacMahon et al. 2005; McKenna et al. 2008; Megibow et al. 2011; Milstein 2008; Morin et al. 2009; Morris et al. 2009; Nelson 2008; Orme et al. 2010; Pierce et al. 2009; Puls et al. 2010; Richardson 2008; Royal and Peterson 2008; Shoemaker et al. 2011; Subhas et al. 2009; van der Lugt 2009; Vanel et al. 2009; Vernooij et al. 2007; Zarzeczny and Caulfield 2012)), radiologic clinical experience and the knowledge and limitations of the applied sequences, a list of reportable IFs has been specifically tailored to the imaging data available (Table 2). Similarly, a list was created exemplarily specifying IFs that should not be reported.
Table 2
Excerpt of the IF-list for the MRI study of the GNC (by February 2015)
Organ system | Report category | IF | Literature |
|---|---|---|---|
Nervous | Acutely relevant—report immediately | Suspected acute stroke | |
Nervous | Acutely relevant—report immediately | Suspected intracranial/intraspinal hemorrhage (including subdural, epidural, subarachnoid, intracerebral, intraventricular hemorrhage) | |
Nervous | Non-acutely relevant—report | Suspected cerebral lesion with edema and/or CSF obstruction and/or midline shift and/or lesion with a size or location prone for complications | |
Nervous | Do not report | Suspected unspecific white matter lesions | |
Vascular | Acutely relevant—report immediately | Suspected aortic dissection | |
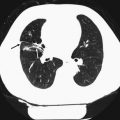
Thoracic | Acutely relevant—report immediately | Suspected pneumothorax | Hegenscheid et al. (2013) |
Thoracic | Non-acutely relevant—report | Suspected lung nodule/mass > 1 cm | |
Abdominal | Non-acutely relevant—report | Suspected adrenal mass > 2 cm | |
Abdominal | Non-acutely relevant—report | Suspected solid or semisolid renal tumor > 2 cm
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|