Imaging modality and references for further information
Scanner
Scan duration (min)
Imaging acquired
n IDP currently available
Examples of available IDP
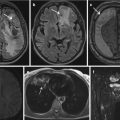
Brain MRI
(UK Biobank 2016)
3.0 T Skyra1
30
T1, T2 FLAIR, SWI, T2*, fMRI, DWI
749
Tissue volumes, activation during fMRI, fractional anisotropy
Cardiac MRI
(UK Biobank 2015b)
1.5 T Magnetom Aera1
20
Cine (long axis, short axis, aorta), tagged, aortic valve flow
30
Cardiac output, ejection fraction, stroke volumes
Abdominal MRI
(UK Biobank 2015a)
1.5 T Magnetom Aera1
10
T1 abdomen, T1 pancreas, liver and pancreas multi-echo, Dixon
5
Percentages of liver fat, fibrosis and haemosiderosis, body composition
DXA
(UK Biobank 2015c)
iDXA2
20
Whole body, thoracolumbar spine, hips, knees
120
Bone area, mineral content and density, lean mass, fat mass
Carotid Doppler US
(UK Biobank 2015d)
5–13 MHz linear array transducer and CardioHealth Station3
10
Video loops in longitudinal and transverse plane, CIMT measures
16
Minimum, maximum and mean CIMT
Participants undergo an approximately 30-min 3.0 T brain MRI (Skyra, Siemens, Erlangen, Germany), which includes structural (T1, T2 fluid-attenuated inversion recovery, susceptibility-weighted imaging and T2*), functional and diffusion imaging (UK Biobank 2016). From these images, UK Biobank generates IDP including measures of volumes of total grey matter, cortical grey matter, total white matter, cerebrospinal fluid and structures such as the thalamus, detailed data on activation and statistical effect sizes in different regions during fMRI tasks and diffusion parameters such as fractional anisotropy in different white matter tracts (UK Biobank 2016; Miller et al. 2016).
A 20-minute non-contrast cardiac MRI is acquired using a 1.5 T Magnetom Aera scanner (Siemens Healthcare, Erlangen, Germany). Sequences include long and short axis cine, aortic distensibility cine, tagging and aortic valve flow images, from which IDP such as cardiac output, ejection fraction and end-diastolic, end-systolic and stroke volumes are calculated and from which a wide range of additional measures are being derived using novel, automated methods (UK Biobank 2015b; Petersen et al. 2013).
Participants are then repositioned within the 1.5 T scanner and undergo a 10-min body MRI. In total, these images cover tissues from the neck to the knees and include a T1 abdomen, T1 pancreas and a liver and pancreas multi-echo sequence. From these images, semiautomated measures of liver fat, fibrosis and haemosiderosis percentages can be made, in addition to body composition measurements of subcutaneous and visceral fat and thigh muscle mass (UK Biobank 2015a; West et al. 2016). Ongoing methodological developments will lead to the derivation of an increasingly wide range of measures.
Carotid Doppler ultrasound images are acquired during a 10-min examination using a 5–13 MHz linear array transducer and a CardioHealth Station (Panasonic, Leicester, UK). Two-dimensional transverse and longitudinal plane images of each carotid artery are saved as cine loops, followed by two measures of intima-media thickness per carotid artery. From these images, mean, minimum and maximum calculations of carotid intima-media thickness are generated, and additional measures of plaque characteristics will follow (UK Biobank 2015d).
DXA images of the whole body, thoracolumbar spine, hips and knees are acquired using an iDXA scanner (GE-Lunar, Wisconsin, USA). The scanner automatically generates multiple IDP of the bone area, mineral content and density and body composition measures of lean and fat mass (UK Biobank 2015c).
Descriptions of all available IDP from each modality, and non-imaging variables, are available from the UK Biobank showcase (http://www.ukbiobank.ac.uk/data-showcase).
2 UK Biobank IF Protocol
2.1 Development of the UK Biobank IF Protocol
Incidental findings (IF) are findings deemed beyond the aims of a study (Wolf et al. 2008). IF are particularly pertinent to the UK Biobank Imaging Study given the nature of IF which may be identifiable on multimodal imaging of 100,000 largely asymptomatic participants. The handling of IF in research imaging is the subject of widespread debates (Gibson et al. 2016 In Press), and while there is no ‘one-size-fits-all’ approach to detecting and feeding back IF, researchers should anticipate IF and design appropriate IF handling policies (Medical Research Council and Wellcome Trust 2014).
The UK Biobank IF protocol was developed following an extensive process which involved reviewing existing policies for feedback of findings to UK Biobank participants, published evidence and guidance on IF, received external legal advice on the scope of the duty of care and consultations with the independent UK Biobank Ethics and Governance Council, UK Biobank’s major funders (Wellcome Trust and Medical Research Council) and with the Royal College of Radiologists and the Society and College of Radiographers. In addition, UK Biobank sought to learn from the experiences and approaches taken to handling IF used by several other large-scale research imaging projects, including the German National Cohort, the Rotterdam Scan Study, the Multi-Ethnic Study of Atherosclerosis (MESA), and the Reykjavik Heart Study. UK Biobank also consulted with relevant experts to explore the legal and ethical factors which were applicable to the development of the IF protocol.
The UK Biobank IF protocol was developed from first principles as a pragmatic protocol that could be implemented on a large scale with the objective of striking the optimum balance of most net benefit and least net harm to 100,000 largely asymptomatic participants (UK Biobank 2015e). Under this protocol, participants only receive feedback in specific, limited circumstances: when a radiographer identifies a potentially serious IF during the acquisition or quality assessment of images during the imaging visit and a radiologist subsequently confirms the presence of a potentially serious IF. UK Biobank defines a potentially serious imaging IF as ‘as a finding which indicates the possibility of a condition which, if confirmed, would carry a real prospect of seriously threatening life span, or of having a substantial impact on major body functions or quality of life.’
2.2 Consent Processes
Before attending the imaging centre, UK Biobank provides participants with an information leaflet which includes a description of the IF protocol and what they should and should not reasonably expect (UK Biobank 2014b).
The information leaflet explains that the scans are not intended to diagnose an illness or identify a particular abnormality and that they will not be looked at routinely by doctors. Participants are informed that if, during the scan, the radiographer notices something which they think may be serious, only then will the scan be reviewed by a doctor; if the doctor thinks there may be a potentially serious finding, the participant and their GP will be informed. The leaflet gives examples of IF which would be fed back to participants (a tumour) and those which would not (gallstones or a simple cyst).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree