Fig. 1
Target volume definition of whole pelvis irradiation. Upper row CTV 3D-four-field planning. Lower row CTV IMRT planning
Finally, modern radiotherapy techniques such as IMRT (intensity modulated radiotherapy) required new approaches for pelvic target volume definition as well as conceptions for sparing organs at risk.
Whereas for several other solid pelvic neoplasms (for example rectal cancer), detailed studies regarding the distribution of locoregional failures are available, there are hardly such data for prostate cancer.
This is related to the fact that—for decades—the major endpoint for outcome definition after curative treatment of prostate cancer is biochemical failure rather than patho-anatomically defined failure. Thus, in the past, it was not exactly shown where and how often patients with prostate cancer finally experience local, locoregional, and distant failure. In addition, the rapid initiation of hormonal ablation therapy after biochemical relapse often had blurred the further topographic attribution of the individual relapse.
Therefore, available topographic data about microscopic pelvic or lymph node involvements in prostate cancer had to be considered since IMRT planning allowed and required individualized target volumes.
3 Surgical Lymphadenectomy Data
Prostate cancer patients who are treated by radical prostatovesiculectomy are mostly undergoing simultaneous pelvic lymphadenectomy. Up to now, the therapeutic importance of a lymphadenectomy procedure regarding potential survival benefits is not finally clear. However, there are some lines of evidence, that progression-free survival is improved when lymphadenectomy is performed (Allaf et al. 2004; Bader et al. 2003; Cheng et al. 2001; Hull et al. 2002).
Aside of its questionable therapeutic role, lymphadenectomy has to be understood as a staging procedure followed by potential relevant therapy recommendations.
However, there are relevant differences with regard to detailed lymphadenectomy modalities. In general, the anatomic extension of pelvic lymph node surgery must be distinguished between “minimal/limited,” “modified/standard,” and “extended” lymphadenectomy. The minimal lymphadenectomy technique only includes the obturator fossa region, and the standard technique furthermore includes the lymphatic area along the external iliac veins, whereas the extended lymphadenectomy area additionally contains all lymph nodes along external, internal, and common iliac vessels (Fig. 2).
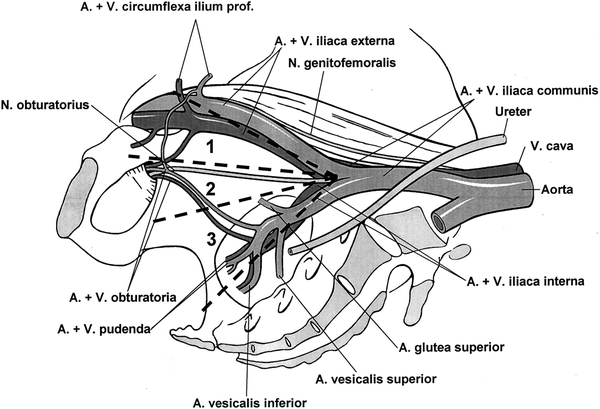

Fig. 2
Lymphadenectomy techniques; 1 + 2 = modified (standard), 2 = minimal, 1 + 2 + 3 = extended lymphadenectomy (Bader et al. 2002)
Derived from large lymphadenectomy series in the recent years with precise histopathological work-up procedures (Allaf et al. 2004; Bader et al. 2002; Weckermann et al. 2005, 2006; Burkhard et al. 2002, 2005; Heidenreich et al. 2002; Briganti et al. 2006, 2007; Shariat et al. 2003; Pagliarulo et al. 2006; Ferrari et al. 2006; Miyake et al. 2007; Stone et al. 1997; Joslyn and Konety 2006) following key conclusions can be drawn:
the risk of locoregional seeding is higher than previously assumed
only for patients at a very low risk for lymph node involvement the procedure of lymphadenectomy seems to be dispensable with regard to morbidity or questionable benefit
at least 10 lymph nodes should be removed when lymphadenectomy is performed (Briganti et al. 2006; Joslyn and Konety 2006)
rates of lymph node involvement are rising both by risk profile and by anatomic extension of lymphadenectomy
the individual lymphatic drainage in prostate cancer patients is more variable than previously suspected
From a radiation oncologist’s view point the lack of information on microscopic lymph node involvement is of special importance with regard to individual radiotherapy treatment decisions—aiming in at least being comparable to clinical outcomes of surgical strategies.
4 Sentinel Lymph Nodes in Prostate Cancer
Weckermann and co-workers developed the prostate sentinel concept using surgical data from more than 2,000 patients (Wawroschek et al. 2003; Weckermann et al. 2007; Holl et al. 2009). Based on a concise histopathological work-up of gamma probe detected sentinel lymph nodes a modified lymphadenectomy procedure was developed. In summary, their findings suggest that a sentinel-guided lymphadenectomy achieves comparable staging results when compared to an extended lymphadenectomy procedure in terms of sensitivity and specificity associated with less morbidity. Data from other groups are in perfect accordance with the results of Weckermann et al. (Bastide et al. 2009; Fukuda et al. 2007; Jeschke et al. 2008).
In an own series on 61 high-risk prostate cancer patients, a SPECT-CT-based sentinel-imaging concept was tested regarding the feasibility for individualized pelvic IMRT planning (Ganswindt et al. 2007, 2011). Although power of the analysis is somewhat limited by the fact that a histological work-up is inherently impossible, the observations regarding number, location, and anatomical distribution of sentinel lymph nodes were very similar to the available surgical sentinel data.
A key result is the fact that up to 30 % of putatively involved lymph nodes would have been missed when standardized radiation portal or standard lymphadenectomy areas would have been used (Fig. 3a, b). Thus, the following key conclusions of the sentinel node concept in prostate cancer can be drawn:
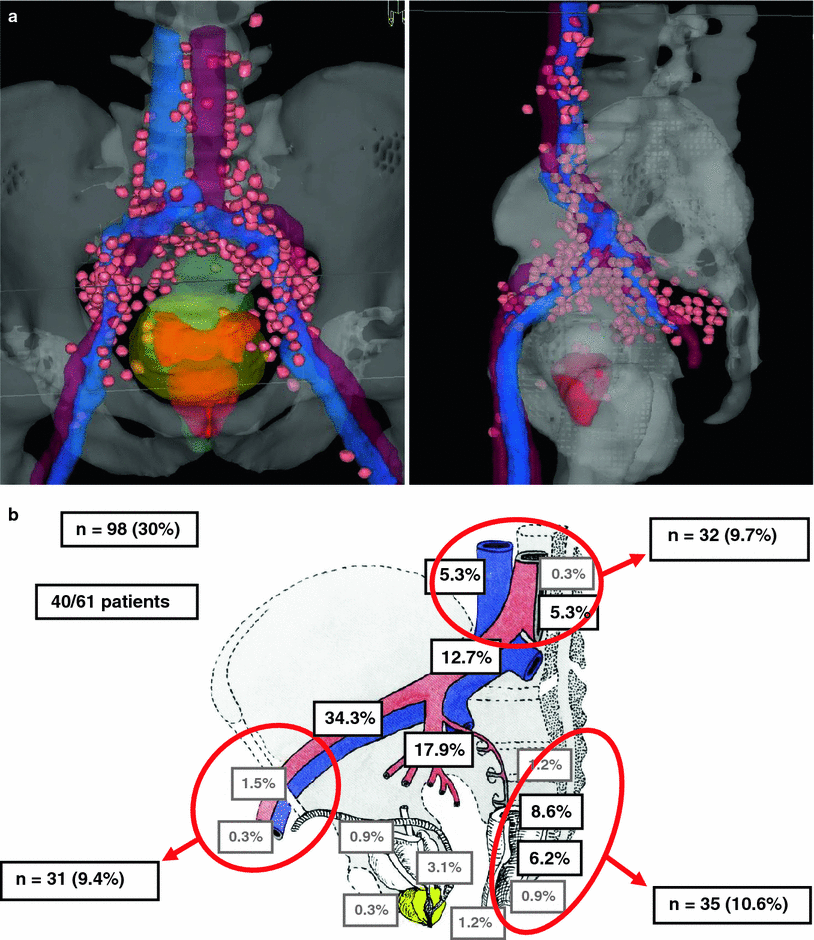

Fig. 3
a Cumulative sentinel lymph node distribution (virtual data set) in 61 patients (left view from ventral above; right view from the left side; sentinel nodes = pink, prostate = red, bladder = green, vessels = blue/red) (Ganswindt et al. 2011). b Areas and anatomical distributions of sentinel nodes with a potential geographic miss. A geographic miss was observed in 98/324 (30 %) sentinel lymph nodes in 40/61 patients (65.6 %) (Ganswindt et al. 2011)
1.
There is a considerable inter-individual pelvic drainage with a highly complex and variable lymph node architecture
2.
Contrary to most other solid tumors in most cases more than one sentinel node is detectable
3.
The sentinel node concept has a high sensitivity (up to 98 %) and specificity—the rate of false negative but histological positive sentinel nodes is in the range of 6 % (Weckermann et al. 2007; Holl et al. 2009).
Lymph nodes next to histopathological positive sentinel nodes often show microscopic lymph node involvement without being sentinel positive
Macroscopic lymph node involvement decreases sensitivity and specificity of the sentinel method—especially with increasing risk profile
“Sentinel-only” imaging data correlate well with surgical data—partially with the exception of regions with close proximity to bladder and seminal vesicles (potentially due to bladder inside radio-nuclide accumulation)
Risk of a geographic miss is predominant in the perirectal and sacral lymphatic drainage areas, followed by the regions along the external iliac chain and high iliac/paraortic nodes
5 Guidelines for Pelvic Target Volume Definition
To overcome the significant variations in the definition of pelvic lymph nodes volumes in the past leading to possible geometrical misses, the RTOG genitourinary radiation oncology group prepared a consensus paper on pelvic lymph node volumes for high-risk prostate cancer patients in 2009 (Lawton et al. 2009). It contains a concise summary of all available data on the prostate lymphatic drainage including surgical and sentinel-guided lymphadenectomy series as well as the results derived from patterns of relapse studies after the use of several radiation volume approaches (Roach et al. 2006).
Key recommendations are:
1.
The inclusion of following adjuvant areas in high-risk prostate cancer patients: “distal common iliac, presacral lymph nodes (S1–S3), external iliac lymph nodes, internal iliac lymph nodes, and obturator lymph nodes.”
2.
The lymph node clinical target volume include the vessels (artery and vein) and a 7 mm radial margin being careful to “carve out” bowel, bladder, bone, and muscle.
3.
Volumes begin at the L5/S1 interspace and end at the superior aspect of the pubic bone.
4.
External iliac contours stop at top of femoral heads (boney landmark for inguinal ligament), obturator contours stop at top of symphysis pubis.
In addition to the RTOG consensus paper, the contouring guidelines are at present available online (http://www.rtog.org/CoreLab/ContouringAtlases/ProstatePelvicLymphNodes.aspx).
Irrespectively of these standardized target volume recommendation, any individual diagnostic finding (including sentinel, MRI- or PET data) on suspicious lymph nodes or anatomic abnormalities in individual patients should possibly be taken into account during treatment planning especially when treatment is performed by highly conformal techniques (Meijer et al. 2013).
6 Treatment Planning 3D versus IMRT
Until today, numerous planning studies comparing 3D conformal radiotherapy with IMRT for the treatment of pelvic nodes in prostate cancer patients exist (Ganswindt et al. 2005b, 2007; Nutting et al. 2000; Luxton et al. 2004; Sanguineti et al. 2006). Due to the rapid implementation of IMRT into clinical practice, valid prospective randomized clinical trials comparing 3D versus IMRT in regard to clinical outcome and toxicities are missing and will not be available in the future.
However, approximately all planning studies based on equal target volumes indicate a superiority of IMRT in terms an improved sparing of organs at risk (bladder, small bowel, rectum, bone), especially for high-radiation dose areas (Fig. 4). In selected cases, target volume coverage may be worse—depending on the individual constraints chosen.
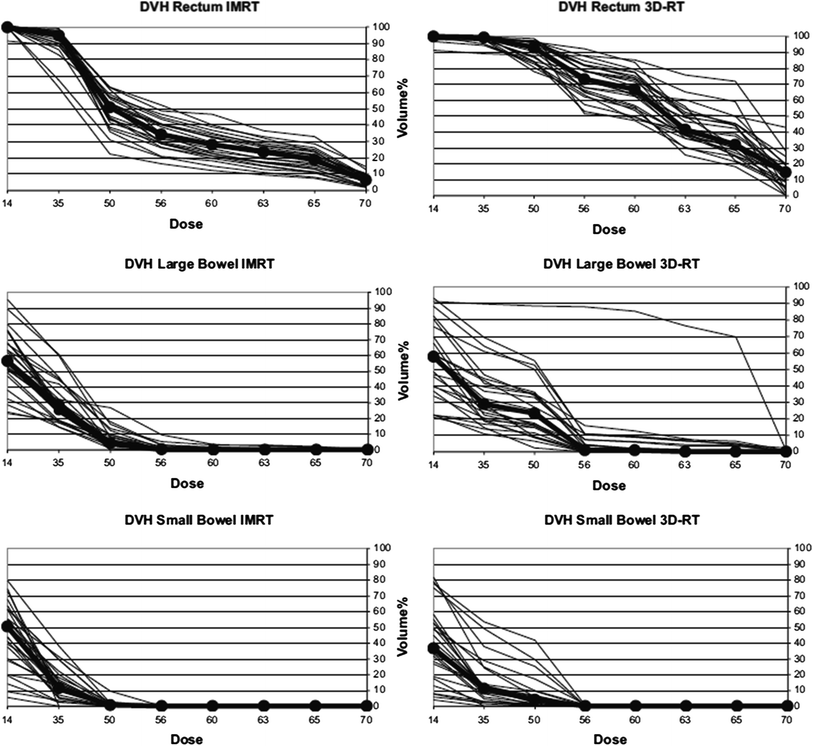

Fig. 4
3D conformal radiotherapy versus IMRT dose-volume histogram comparison of small bowel, large bowel, and rectum. Cumulative dose-volume-histograms of 25 patients are shown. Solid lines denote patients 1–25; lines connecting dots denote median of 25 patients. (Ganswindt et al. 2007)
In summary, the use of IMRT allows to apply higher doses to the prostate without increasing acute or late toxicities and in parallel allows for an highly conformal dose coverage of pelvic lymph nodes with low acute and late toxicity profiles (Ganswindt et al. 2007; Guckenberger et al. 2008; Sharma et al. 2011; Zelefsky et al. 2012; Vora et al. 2007; Al-Mamgani et al. 2009; Eade et al. 2008).
7 IMRT Planning and Treatment
Pelvic clinical target volume (CTV) delineation should be routinely performed on a CT planning data set in exact treatment position. Usually 3- or 5-mm CT slices are applied, depending on different requirements for dose calculation algorithms being used in a given institution.
In selected cases, a prone position (with belly board) allows improved sparing of small bowel especially when large parts of small bowel are located within the distal pelvic area. However, in general, a supine position results in a more stable patient position, still with adequate sparing of the small bowels. Furthermore, it allows the use of image guidance modalities including ultrasound imaging which is impossible when patients are positioned in a prone position.
Planning and treatment should be performed with nearly empty rectum and comfortably filled bladder. Bladder filling improves sparing of relevant bladder wall and bowel volumes. The irradiation with an empty rectum seems to be associated with better clinical outcomes and less rectal toxicities (Heemsbergen et al. 2007; Engels et al. 2009; Reddy et al. 2009; Jain et al. 2012).
Reasonable safety margins from CTV to PTV are in the range of 5–10 mm; however, the choice of safety margins depends substantially on individual positioning and image guidance strategy being implemented in the given institution. Besides influencing bladder and rectal filling modern image guidance tools as cone beam computed tomography, ultrasound or fiducial markers allow to correct the related position of the prostate during daily radiation treatment. In this regard, it is of special importance not only to focus on the moving prostate position, but also to sufficiently cover the rather immobile pelvic node CTV (Ferjani et al. 2013; Adamczyk et al. 2013; Xia et al. 2010).
8 Dose Prescriptions
Historically, the RTOG used 50.4 Gy to the pelvic nodes followed by a boost to the prostate to cumulative 70.2 Gy (single doses 1.8 Gy, 5/week) and EORTC trial prescribed 50 Gy (pelvic nodes) and 70 Gy (prostate), respectively, but in single doses of 2.0 Gy, 5/week.
Currently, a cumulative dose of 70 Gy to the prostate in a high-risk constellation is considered to be suboptimal (Hanks et al. 2000; Pollack et al. 2002; Peeters et al. 2006; Ganswindt et al. 2005a). Thus, when using pelvic IMRT, a cumulative dose of 74–76 Gy to the prostate region seems advisable. Several trials have shown that this is feasible without increased toxicity profiles (Guckenberger et al. 2008; Zapatero et al. 2005).
One of the key features of IMRT technique is the possibility to employ different dose levels simultaneously (simultaneous integrated boosts [SIB]) within one treated volume. Thus, a dose prescription to the pelvic nodes of 50.4 Gy (single dose 1.8 Gy per fraction) and simultaneously of 2.0 Gy single dose to the prostate is commonly used in clinical practice at present. After these first 28 fractions with SIB, irradiation is continued by a different boost treatment plan of 14 Gy (18 Gy, respectively) without any dose split.
Dose constraints to the given organs at risk (bladder, small bowel, rectum, colon, bone) should aim for a minimum dose adherent to the recent QUANTEC data (Jackson et al. 2010). Thus, each individual IMRT plan needs to be reviewed consequently in order to optimize irradiated normal tissue volumes.
In how far different IMRT techniques [static (step and shoot) techniques and dynamic techniques (sliding windows with or without simultaneous gantry rotation, tomotherapy)] may be advantageous has not been finally determined in clinical trials. Based on theoretical assumptions, fast dynamic techniques (intensity modulated arc therapy, IMAT, “Rapid Arc”) may turn out to be advantageous in terms of shortened treatment time and thus reduced intra-fractional organ movements. However, on the other hand, the exact dosimetry and quality assurance of highly modulated IMRT may impose new and unexpected problems.
In how far particle therapy may improve the treatment of prostate cancer pelvic nodes has not been determined at present (Sheets et al. 2012). The fact that many pelvic organs are mobile, air filled, and display extreme variations in organ filling, indicate that these targets may not be optimal targets for particle approaches.
In parallel with the implementation of standard IMRT techniques covering prostate and the adjuvant pelvic lymph nodes with normal fractionation schemata, several small series using a moderate hypo-fractionation to the prostate in combination with pelvic node irradiation were reported (Alongi et al. 2012; Norkus et al. 2013; Krause et al. 2012). Guckenberger recently reported on 41 patients treated with 32 × 2.3 Gy SIB to the prostate, according to 80 Gy 2 Gy-equivalent. The 5-year freedom from biochemical failure (FFBF) was 78 % for high-risk disease with low rates of observed toxicity by the use of IMRT and accurate image guided radiotherapy (IGRT) (Guckenberger et al. 2014).
9 IMRT Techniques in Node-positive Patients
Widespread PSA screening has certainly led to increased diagnosis of lower risk prostate cancer and recent studies found a dropping incidence of lymph node metastases (even less than 10 %) (Nguyen et al. 2009; Paul et al. 2010). However, the number of patients with nodal involvement at baseline remains high in subgroups (up to 30–40 % in high-risk patients (Crehange et al. 2012))—particularly when the extension of lymphadenectomy and pathological work-up procedures are taken into account (Briganti et al. 2008). Furthermore, as mentioned above, the real risk of lymphonodular seeding is higher than previously assumed (Weckermann et al. 2006; Heidenreich et al. 2002; Briganti et al. 2006; Shariat et al. 2003; Pagliarulo et al. 2006; Miyake et al. 2007).
At a minimum, there are three different clinical start points regarding node-positive prostate cancer patients and radiation treatment approaches:
Firstly,
patients with prostate cancer at the time of first diagnosis and synchronously macroscopic lymph node metastasis (cN1) without any surgery.
Secondly,
patients with newly diagnosed prostate cancer and synchronously clinical or subclinical lymphatic involvement, but after surgery/lymphadenectomy (pN1).
Thirdly,
patients after primary treatment of prostate cancer and metachronously loco-regional relapse at a site of the pelvic lymphatic region (rN1).
Provided that lymph node involvement is considered as origin, and not merely as surrogate for distant metastatic spread, a loco-regional approach may be appropriate.
Ad 1 (cN1 patients):
Derived from some older retrospective series (Sands et al. 1995; Whittington et al. 1995; Wiegel and Bressel 1995) and a number of subset-analyses of RTOG trials (75-06 (Hanks et al. 1998), 85-31 (Lawton et al. 2005) and 92-02 (Hanks et al. 2003)) there is strong evidence that also lymph node-positive patients could achieve excellent local control rates when pelvic irradiation is combined with hormone therapy. Thereby, the RTOG 85-31 subset analysis in 173 N1 patients revealed biochemical progression-free survival rates at 5/9 years of 33 %/4 % and 54 %/10 %, respectively, in favor of the combined treatment arm. Yet while the previously used “box technique” did not allow higher radiation doses to involved lymph nodes due to bowel toxicity, the dose required to treat with a curative intent positive nodes is higher (i.e. ≥60 Gy).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree