Embolization of arteries. This may occur in a variety of conditions. It is seen, for example, in certain hemoglobinopathies, such as sickle cell disease, in which arteries are occluded by abnormal red blood cells; in decompression states of dysbaric conditions, such as caisson disease, in which embolization by nitrogen bubbles occurs; or in chronic alcoholism and pancreatitis, when fat particles embolize arteries.
Vasculitis. Inflammation of the blood vessels may lead to interruption of the supply of arterial blood to the bone, as seen in collagen disorders such as systemic lupus erythematosus (SLE). However, the use of steroids in these conditions is a major cofactor and may be even more contributing determinant than the primary disease.
Abnormal accumulation of cells. In Gaucher disease, which is characterized by the abnormal accumulation of lipid-containing histiocytes in the bone marrow, or after steroid therapy, which can lead to an increase of fat cells, sinusoidal blood flow may be compromised, resulting in a deprivation of blood supply to the bone.
Elevated intraosseous pressure. This theory, championed by Hungerford and Lennox, suggests that any acute or chronic pathologic process that results in increased pressure within the femoral head (which is essentially a sphere of cancellous bone, marrow, and fat surrounded by a cortical shell) may compromise the blood flow and lead to osteonecrosis. The use of steroids and alcohol abuse falls into this category, as well as chronic tobacco use, although the latter addiction plays relatively minor role compared with the two formerly mentioned.
Inhibition of angiogenesis. Osteonecrosis may result from compromise of normal angiogenesis that occurs consistently in bone tissue. Smith and coworkers recently introduced this new hypothesis, supported by the fact that a number of drugs and mediators, including glucocorticoids, interferons, and other endogenously produced cytokines, inhibit angiogenesis. A similar effect was observed in the angiographic studies of the femoral head after the administration of steroids.
Mechanical stress. This causative factor was occasionally attributed to nontraumatic osteonecrosis of the femoral head. The weight-bearing segment of the femoral head is the anterior-superior quadrant and, therefore, is under a large mechanical strain. Occlusion of the vessels in this region of the femoral head might be the result of cartilage breakdown secondary to excessive mechanical stress. Support for this hypothesis stems from experiments on rats by Iwasaki et al. and Suehiro et al.
Radiation exposure. Exposure to radiation may result in damage to the vascularity of a bone.
Idiopathic. Often, no definite cause can be established, as in the case of certain conditions such as Legg-Calvé-Perthes disease involving the femoral head or Freiberg disease affecting the head of the second metatarsal. This diagnosis is usually made when the osteonecrosis affects only one bone and should only be entertained when all other etiologic factors are excluded.
is the subject of an ongoing investigation. Single nucleotide polymorphisms have been noted in a number of genes that may be associated with osteonecrosis. It has been argued that endothelial nitric oxide synthase is an important player in the development of this condition. Nitric oxide may have beneficial effects on three systems involved in osteonecrosis, namely, skeletal, vascular, and thrombotic. Each of these may be a target for proposed mechanisms of pathogenesis. A comparative analysis of the 26-base pair repeats polymorphism in intron 4 and the Glu298Asp polymorphism in exon 7 of the eNOS gene in patients with idiopathic, steroid-induced, alcohol-induced, and normal control subjects was performed. The frequency of the homozygous 4a allele was found to be higher in patients with idiopathic osteonecrosis compared with control subjects, and the frequency of the 4a/b allele was found to be higher in all types of osteonecrosis when compared with control subjects. The 4a allele is known to be associated with reduced synthesis of endothelial nitric oxide synthase, suggesting that nitric oxide may play a protective role against the development of osteonecrosis.
Table 13.1 DISEASES OR CONDITIONS ASSOCIATED WITH OR LEADING TO OSTEONECROSIS | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||
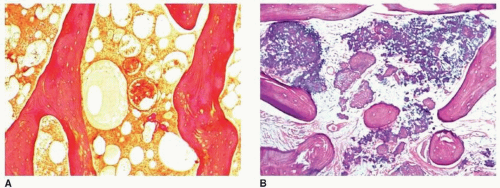
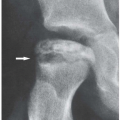
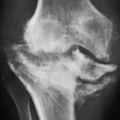
earliest radiographic sign of this condition is the presence of a radiolucent crescent, which may be seen as early as 4 weeks after the initial injury. This phenomenon, as Norman and Bullough have pointed out, is secondary to the subchondral structural collapse of the necrotic segment and is visible as a narrow radiolucent line parallel to the articular surface of the bone. Radiographically, the sign is most easily demonstrated on the frog-lateral view of the hip (Figs. 13.5 and 13.6). Because the necrotic process most of the time does not affect the articular cartilage, the width of the joint space (i.e., the radiographic joint space: the width of the articular cartilage of adjoining bones plus the actual joint cavity) is preserved. Preservation of the joint space helps to differentiate this condition from osteoarthritis. In its later stage, osteonecrosis can be readily identified on the anteroposterior view of the hip by a flattening of the articular surface and the dense appearance of the femoral head (Figs. 13.7 and 13.8). The density is secondary to the compression of bony trabeculae after a microfracture of the nonviable bone, calcification of the dendritic marrow, and repair of the necrotic area by the deposition of a new bone, the so-called creeping substitution. CT examination frequently helps to delineate the details of this condition (Figs. 13.9 and 13.10). Ficat and Arlet proposed a
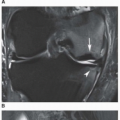
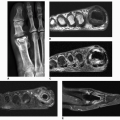
classification system of osteonecrosis of the femoral head consisting of four stages, based on radiographic, hemodynamic, and symptomatic criteria (Table 13.2). A significant breakthrough in identifying osteonecrosis in patients who had normal bone scan and normal conventional radiographs was achieved with MRI. Currently, this modality is considered the most sensitive and specific for the diagnosis and evaluation of osteonecrosis (Fig. 13.11). Its characteristic MRI appearance consists of a serpentine band of low-signal intensity rim in the femoral head (Fig. 13.12A). This rim corresponds to the interface of repair between ischemic and normal bone consisting mainly of sclerosis and fibrosis. On T2-weighted images, a second inner rim of high signal has been observed (the double-line sign) (Fig. 13.12B). It is believed that this appearance represents fibrovascular tissue in the reparative zone. Many authors hypothesize that this finding is pathognomonic for osteonecrosis. Other authors have played down the importance of this finding, claiming that it may be largely artifactual, representing the so-called chemical shift. Bone marrow edema and joint effusion are frequently associated with osteonecrosis (Fig. 13.12C). Once the subchondral fracture occurs, the femoral head will collapse (Fig. 13.12D), and eventually, the hip joint will develop secondary osteoarthritis. Intravenous injection of gadolinium can help to delineate the extension of the osteonecrosis and determine if there are areas of residual viable tissue (Fig. 13.12E). Several reports have established the diagnostic sensitivity of MRI in the early stages of osteonecrosis, when radiographic changes are not yet apparent or are nonspecific. MRI has been shown to have 97% sensitivity in differentiating osteonecrotic femoral head from normal femoral head and 85% sensitivity in differentiating osteonecrotic femoral head from other disorders of the femoral head, with an overall sensitivity of 91%. MRI appears to be a better predictive test for subsequent femoral head collapse than radionuclide bone scans. The narrow bandlike area of low signal intensity that traverses the femoral head in midcoronal sections present on MRI was a significant indicator of subsequent collapses.
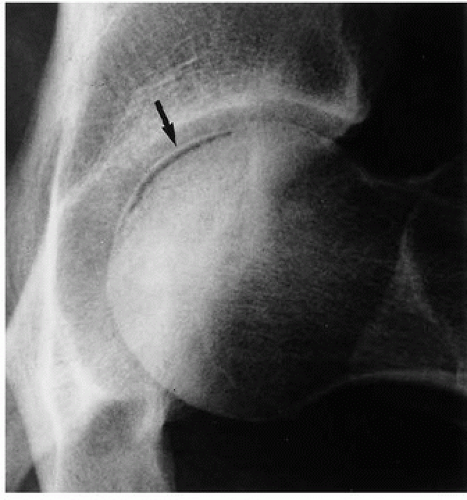 Figure 13.5 ▪ Osteonecrosis of the femoral head. The froglateral view of the left hip shows the crescent sign (arrow) in a 45-year-old woman who sustained a hip dislocation 5 weeks earlier. |
Table 13.2 OSTEONECROSIS OF FEMORAL HEAD: CORRELATION OF CLINICAL SYMPTOMS AND IMAGING FINDINGS WITH HISTOPATHOLOGIC CHANGES BASED ON FICAT AND ARLET CLASSIFICATION | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
 Figure 13.11 ▪ MRI of osteonecrosis of the femoral head. Coronal T2-weighted MRI in an 18-year-old woman with SLE demonstrates a focal area of osteonecrosis of the femoral head.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|