Fig. 12.1
Various (spherical, needle, cylindrical) applicators for the INTRABEAM system
12.2 Surgical Applications
Between January 2008 and July 2011, a total of 223 IORT procedures were performed in Mannheim, including 150 in patients with breast cancer and 73 in those undergoing surgery for other cancers (Fig. 12.2). After IORT for breast cancer, the most frequent procedures were kypho-IORT (30 cases) and IORT for sarcomas and recurrent rectal cancer (25 and 11 cases, respectively).
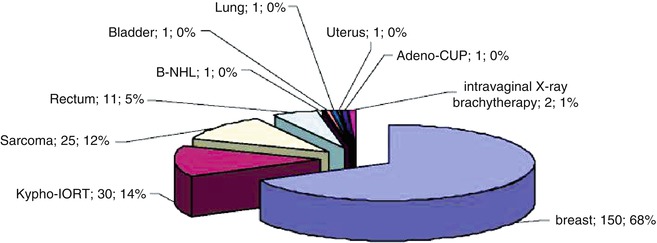

Fig. 12.2
IORT in Mannheim between January 2008 and July 2011
Sarcomas are rare cancers, accounting for only 1–3 % of all cancers. They are a very heterogeneous group of tumours, roughly divided into bone and soft tissue sarcomas. Soft tissue sarcomas are the largest subgroup and most often arise in the extremities and trunk. The mainstay of treatment is surgery. Nevertheless, the management of soft tissue sarcoma has evolved from solely surgical treatment to an interdisciplinary multimodal approach including surgery, chemotherapy and radiotherapy. External beam radiotherapy (EBRT) applied pre- or postoperatively improves the probability of local control and results in cure rates that are comparable to those achieved with more extensive resections or amputations (Rosenberg et al. 1982). IORT alone or followed by postoperative EBRT is also a reasonable radiotherapy treatment option. Lehnert et al. (2000) observed a 40 % reduction in local recurrence after IORT in patients with soft tissue sarcomas of the extremities, the trunk or the retroperitoneum in comparison with patients treated with surgery alone. One randomised study, by Sindelar et al. (1993), compared combined IORT and postoperative EBRT versus EBRT alone and observed a local recurrence rate of 40 % versus 80 % without any difference in overall survival after 5 years.
Among the 25 patients with sarcoma who we treated with IORT, one was an 80-year-old with a recurrent liposarcoma of the retroperitoneum. The initial diagnosis of retroperitoneal liposarcoma had been made 6 years previously. The patient initially underwent tumour resection without adjuvant therapy. After diagnosis of relapse, preoperative EBRT was first administered. Due to a lack of response, EBRT was stopped after 27 Gy and a wide excision with IORT in the field of the residual tumour (applicator size 5 cm; dose 12 Gy at the applicator surface) was performed (Fig. 12.3).
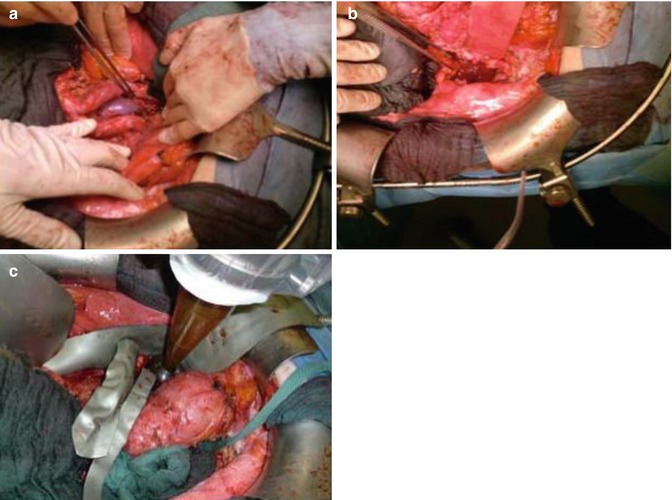

Fig. 12.3
(a, b) Retroperitoneal operating field after wide excision of a relapsed liposarcoma. (c) Positioning of the spherical applicator within the range of the microscopic residual tumour (R1)
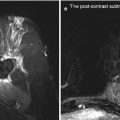
Another example was a 56-year-old patient with a high-grade sarcoma of the right thigh. In this case, multimodal treatment was delivered. The patient first underwent a wide excision with IORT (applicator size 5 cm; dose 5 Gy), followed by EBRT with 60 Gy (Fig. 12.4).
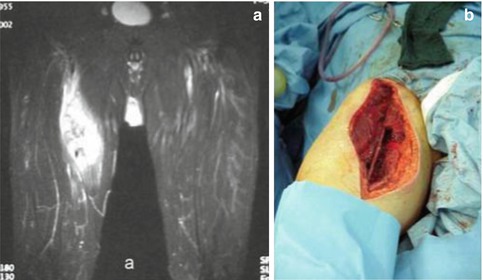

Fig. 12.4
(a) Preoperative coronal MRI showing a high-grade sarcoma of the right thigh. (b) Wound after resection of the tumour, before insertion of the applicator
The incidence of rectal cancer is 15–25 cases per 100,000 inhabitants. Preoperative radio-chemotherapy followed by surgery by means of improved techniques has become the standard of care for locally advanced tumours because of improved local control and the effect of downsizing/-staging (Ferenschild et al. 2006). Recurrent rectal cancer, however, remains a very serious problem. Between 3 and 15 % of patients develop a local relapse, and without treatment the median overall survival for patients with recurrent rectal cancer is only 6 months (McDermott et al. 1985; Welch and Donaldson 1978). A particular challenge is posed by previously irradiated patients, because the required high dose cannot be administered again. Moreover, resection margin involvement has been demonstrated to be the most important prognostic factor in patients undergoing surgery for recurrent rectal cancer, but the likelihood of negative margins after resection is low.
Valentini et al. (2006) reported results of treatment with EBRT and concurrent chemotherapy with or without salvage surgery in 59 patients with previously irradiated recurrent rectal cancer. Median dosage was 30 Gy followed by a boost of 10.8 Gy (1.2 Gy twice daily with a minimum 6-h interval). During the radiation treatment, concurrent chemotherapy was delivered (5-fluorouracil, protracted intravenous infusion, 225 mg/m2/day, 7 days per week). The overall 5-year survival was 39 %. Another treatment option for previously irradiated patients with local relapse is surgery combined with IORT. Suzuki et al. (1995) described the outcome of 106 patients who underwent palliative resection of locally relapsed disease. Forty-two patients received IORT as a component of treatment (mostly 15–20 Gy) and 41 received EBRT (mostly 45 Gy). Patients receiving IORT had a 5-year survival rate of 19 %, compared with 7 % among those without IORT. An updated Mayo Clinic analysis (Haddock et al. 2001) described 175 patients with locally recurrent colorectal cancer (123 without and 52 with prior EBRT) who underwent IORT. Five-year survival in previously irradiated patients was 12 %. The 3-year local control rate for the same group was 51 %.
Between January 2008 and July 2011, 11 patients at our centre received IORT for the treatment of recurrent rectal cancer. Figure 12.5 shows the intraoperative setting in a 73-year-old patient with metastasised rectal cancer with infiltration of the bladder and the anal skin after initial abdominoperineal rectum extirpation in 2002. A transanal excision was performed in combination with IORT (dose 4 Gy to a tissue depth of 5 mm, applicator size 4 cm) as well as a radical cystectomy. In the future a flat applicator will be available for treatment of rectal cancer recurrences while sparing the surrounding tissue. The flat design of the new applicator will allow irradiation to a depth of 0.1–1.0 cm without scattering dose to the surrounding organs at risk.
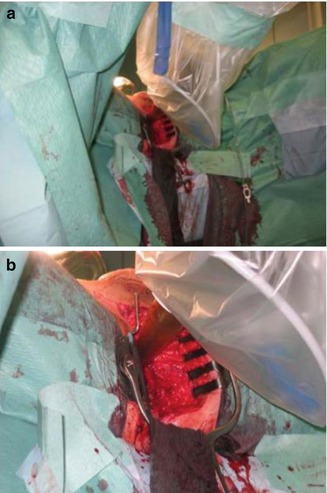

Fig. 12.5
(a, b) IORT after transanal excision and radical cystectomy with the positioned applicator inside the operative field (dose 4 Gy in a tissue depth of 5 mm, applicator size 4 cm)
All our experience in use of IORT with the INTRABEAM system for the treatment of recurrent rectal cancer or sarcomas concerns individual cases. As yet, no clinical trials have been performed on IORT in such patients.
12.3 Kypho-IORT – IORT During Kyphoplasty
12.3.1 Indications
The skeletal system is the most common site of metastases, being affected in up to 30 % of all cancer patients (Hage et al. 2000). The leading focus is the vertebral column with an incidence of 30–70 % (Wong et al. 1990; Boland et al. 1982; Harrington 1986). Most patients (70–90 %) suffer from severe axial pain. Besides this, vertebral metastases can cause pathological fracture and neurological dysfunction by spinal cord compression (Janjan 1997; Gilbert et al. 1978; Smith et al. 2000). Although the median overall survival of patients with advanced cancer is limited, the survival time of patients with bone metastases varies depending on the primary tumour. Therefore it is necessary to optimise the palliative treatment to achieve a high quality of life.
For patients with spinal metastases but without spinal cord compression and pain or impeding or existing instability, surgery and external beam radiotherapy (EBRT) are frequently used treatment options. Furthermore, it is well established that decompressive surgery with adjuvant radiotherapy exerts analgesic effects with good local tumour control in patients with painful spinal metastases (Moulding et al. 2010; Gerszten et al. 2009; van der Linden et al. 2006). Nevertheless, pain relief takes weeks and structural stability, months. For patients with pathological fracture or vertebrae prone to fracture without epidural compression, minimally invasive surgical techniques like kyphoplasty or vertebroplasty have been shown to yield pain reduction and sufficient stabilisation (Halpin et al. 2004; Garfin et al. 2001). However these surgical therapies have no anticancer effect and postoperative radiotherapy is necessary to prevent early regrowth. Disadvantages of this combined therapy include the long treatment period, typically 1–4 weeks, which is often associated with mental and physical strain; in addition, chemotherapy and fractionated radiotherapy are typically not applied simultaneously owing to increased side-effects.
The combination of kyphoplasty and a single dose of IORT (kypho-IORT) attains sufficient stability under sterilisation of the metastasis and reduction of treatment time. Kypho-IORT could therefore be an interesting therapeutic option in the palliative setting. Patients with oligometastases who meet the following eligibility criteria can be considered suitable for kypho-IORT: (a) pathologically or cytologically proven primary tumour; (b) painful or unstable lesions between T4/5 and L5 and (c) Karnofsky index ≥60 %. Our recently published findings (Schneider et al. 2011) indicate that up to one-third of patients with spinal metastases meet these criteria. Simultaneous visceral metastases do not represent an exclusion criterion, but patients with primary bone tumours, soft tissue extension and epidural or intraspinal invasion are excluded. Prior to kypho-IORT, all patients should undergo a physical examination and a CT or MRI scan to evaluate the extension of the metastases.
12.3.2 Physical and Technical Aspects
Prior to the first clinical application, kypho-IORT was performed in a donated body in order to estimate the delivered doses in the surrounding tissue. Two ionisation chambers, one beside the vertebra and one in the spinal cord, were used (Schneider et al. 2011). Measurements revealed a maximum dose of 3.8 Gy in the spinal cord and a dose of 0.2 Gy adjacent to the vertebra. Examples of dose distributions depending on the position of the radiation source are shown in Fig. 12.6.
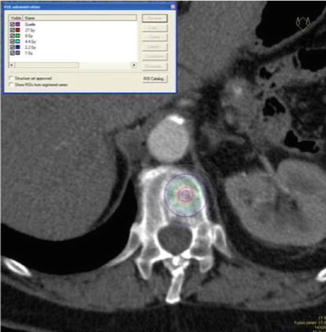

Fig. 12.6
Approximate dose distribution
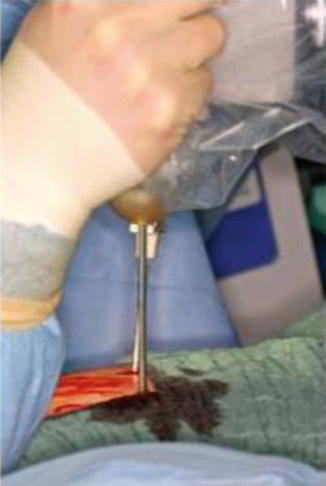
The procedure of balloon kyphoplasty (Medtronic, Kypho, Minnesota, USA) is done according to standard kyphoplasty with minor modifications. All patients are placed in the prone position on a radiolucent table under general anaesthesia. After sterile preparation, the Kirschner wire is inserted; this is followed by application of the working cannula, with a metallic sleeve designed especially for kypho-IORT, which is positioned in the pedicle. After removal of the working cannula, a drill is inserted to create a tract into the vertebra.
To permit use of the INTRABEAM system for kyphoplasty, a specially designed applicator was developed. This applicator (4.1 mm diameter) consists of a stainless steel tube with a tip made of plastic and a plastic head, which is necessary to adapt it to the X-ray source of the INTRABEAM system (Fig. 12.7). The steel tube of the applicator protects the sensitive probe from bending and the plastic tip minimises the absorption.
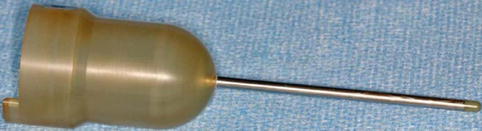

Fig. 12.7
Specially designed needle applicator for kypho-IORT
After removing the burr, the applicator, including the drift tube of the INTRABEAM, is guided through the specially designed metallic sleeve (5 mm diameter, 6 cm length) into the vertebral body (Fig. 12.8). For verification of the correct position of the guiding sleeve and the applicator, biplanar X-rays are used. A single radiation dose of 8 Gy in 10 mm is delivered during 2 min to the centre of the metastasis (Fig. 12.9). After application of radiation, the INTRABEAM system is removed and the working cannula is again inserted while the metallic sleeve remains in place to avoid reflow of the cement. Further steps of kyphoplasty conform to standard kyphoplasty, with inflation and deflation of the balloon followed by cement injection (Fig. 12.10).