US appearance
Origin of hepatic metastases
Hypoechoic metastases
Breast, lung cancer, pancreas cancer, lymphoma
Hyperechoic metastases
Colon cancer, RCC, pancreas islet cell tumor, neuroendocrine carcinoma
Target pattern
Most common in lung cancer. GI origin tumor
Cystic metastases
Mucin-producing tumors (colon, ovary, IPMN)
Necrosis: sarcoma, GIST
Calcified metastases
Mucin-producing tumors (colon, ovary, breast, stomach)
Neuroendocrine, thyroid medullary carcinoma, osteosarcoma
Infiltrative
Breast, lung cancer, melanoma
5.6.2 Computed Tomography
Contrast-enhanced CT is widely used for the detection, characterization, and follow-up of hepatic metastases and a primary imaging tool for the management. Most of the hepatic metastases are supplied by hepatic arteries and do not have a significant supply from portal vein. Therefore, majority of the hepatic metastases are hypovascular than hepatic parenchyma in portal venous phase when hepatic parenchyma is maximally enhanced, and many institutions perform portal venous phase only for the patients of hepatic metastases. However, other phase images have some roles also. On non-contrast images, most of the metastases are low attenuated compared to hepatic parenchyma. Calcifications are clearly visible on non-contrast images. Size of the tumors is sometimes more accurate in non-contrast images because peritumoral shunts or hyperemia does not hinder the measurements. Hypervascular tumors are best seen on arterial phase, and some hypervascular tumors are not clearly visible on portal venous phase. Therefore, adding arterial phase to portal venous phase can increase detection rate of hypervascular metastases. Arterial phase images can be helpful to detect small tumors because they usually do not have central necrosis and hyperdense rim of metastases is well visualized on arterial phase. Hyperdense rim of arterial phase is caused by desmoplastic reaction, inflammation, or shunt due to high blood flow of hypervascular metastases. Common primary tumors with hypervascular metastases include renal cell carcinoma, neuroendocrine tumors, melanoma, pheochromocytoma, choriocarcinoma, and some cases of breast or lung cancer. Majority of hepatic metastases are hypovascular tumors seen as low attenuated lesion after contrast injection. These tumors are well visualized on portal venous phase. Gastrointestinal origin tumors, squamous cell carcinoma, urothelial cell carcinoma, lung, breast, and prostate cancer are common primary tumors for hypovascular metastases. Hypovascularity of these tumors is caused by dense cellularity, fibrosis, or necrosis. Peripheral area of these tumors consists of viable tumor cells and shows no or slight rim enhancement on arterial phase. This area is being thicker and showing slight washout on portal venous or delayed phase (“peripheral washout sign”). Central area of large hypovascular metastases consists of necrosis, hemorrhage, or desmoplastic reaction. Fibrosis due to desmoplastic reaction can show prolonged enhancement, and this finding is common in cases of metastatic adenocarcinoma, cholangiocarcinoma, lymphoma, and epithelioid hemangioendothelioma. As described early in Sect. 5.6.1, cystic metastases can be seen in patients with mucin-producing primary tumors or tumors with prominent necrosis. Some primary tumors are solid, but their hepatic metastases can be cystic because of the large size of metastatic lesions and poor vascularity. Diffuse and infiltrative metastases are very difficult to be differentiated from diffuse liver parenchymal disease such as cirrhosis. Heterogeneous parenchymal attenuation, deformity of hepatic contour, and narrowing of intrahepatic vasculatures are imaging findings of infiltrative metastases of the liver. However, direct invasion of hepatic vessels is uncommon. Lymphangitic metastases are tumor cell infiltration in periportal area, and imaging findings are widening of periportal space and low attenuated area along portal veins on contrast-enhanced images. However, this finding can be observed also in the cases of periportal edema due to various reasons. Pseudocirrhosis is the result of chronic liver injury after chemotherapy and almost exclusively reported in the patients of metastatic breast cancer. Retracted tumor tissue with scarring is the cause of pseudocirrhosis. Capsular retraction with coarse nodules is reported finding of pseudocirrhosis. Other features of liver failure including ascites, splenomegaly, and varices can be accompanied.
5.6.3 Magnetic Resonance Imaging
MRI was a problem-solving tool for hepatic metastases, and a role of MRI for hepatic metastases was characterization of focal hepatic lesions, mainly differentiating metastases from benign lesions such as cysts or hemangiomas. However, diagnostic performance of hemangiomas has been improved, and now MRI shows better sensitivity and specificity comparing to MDCT or PET. Especially, hepatocyte-specific contrast agents (Gd-EOB-DTPB, Gd-BOPTA) can enhance conspicuity and sensitivity of hepatic metastases on hepatobiliary phase images, and now sensitivity of liver MRI with these contrast agents is comparable or better than that of intraoperative US, which was the gold standard previously. Signal intensity of hepatic metastases on non-contrast T1- and T2-weighted images is variable according to the contents of the tumors such as hemorrhage, fibrosis, necrosis, and vascularity. However, in majority of cases, hepatic metastases are seen as iso- to low signal intensity compared to hepatic parenchyma on T1-weighted images and iso- to high signal intensity on T2-weighted images. T1 and T2 relaxation times of metastases are usually longer than normal hepatic parenchyma and shorter than common benign focal lesions such as cysts or hemangiomas. Still, one of the major strengths of MRI over CT is easy differentiation of hepatic metastases from such benign lesions. Heavily T2-weighted images are very helpful, and benign lesions such as cysts or hemangiomas look very bright. Therefore, signal intensities of metastases are lower than those of cysts or hemangiomas on T2-weighted images and higher on T1-weighted images. Peritumoral halo is seen as higher signal rim on T2-weighted images. Hypervascular tumors such as renal cell carcinoma and cystic metastases can be seen as very high signal intensity lesions, and differentiation from cysts or hemangiomas can be difficult in these cases. Intratumoral hemorrhage, coagulative necrosis, melanin, or mucin may be seen as mixed signal intensity on T1-weighted images. Also, T2-weighted images show coagulation necrosis, mucin, and fibrosis as low-signal area in the center of the tumors. Doughnut sign shows a low signal intensity rim surrounding the center of even lower signal intensity, and this pattern is usually observed in cases of large tumors prone to necrosis. Target sign is observed on T2-weighted image, and higher signal intensity due to necrosis is present in central area of large tumors. After contrast injection, vascularity of tumors is similar to that of CT scans, and peripheral washout sign can be observed (see 5.6.2). Lymphangitic metastases may be observed in high signal area along portal veins. On hepatobiliary phase images using hepatocyte-specific contrast agents (Gd-BOPTA or Gd-EOB-DTPA), hepatic metastases should appear low signal intensity (“defect”) comparing to the positive enhancement of hepatic parenchyma. However, there are some reports describing the slightly higher signal intensity in the central area of hepatic metastases at hepatobiliary phase using hepatocyte-specific contrast agents. The cause of this paradoxical phenomenon is unknown, but prominent desmoplastic reaction, large interstitial space, and coagulation necrosis of central area of metastases may retain gadolinium chelates. Diffusion-weighted images (DWI) also can be helpful for detecting and characterizing focal hepatic lesions. DWI has similar performance to hepatobiliary phase images for detecting hepatic metastases, and combining both techniques was reported to be best for the diagnostic performance. MRI can be helpful to differentiate lymphangitic metastasis from periportal edema. Delayed enhancement of periportal fibrosis can be delineated on delayed phase images after gadolinium-based contrast agent injection.
5.7 Lymphomas and Plasmacytomas
5.7.1 Lymphoma
Liver is one of the most commonly involved organs of lymphomas, and majority of the cases are secondary lymphoma. More than 50 % of the patients of lymphoma have hepatic lesions, but primary hepatic lymphoma is rare because the lymphatic tissue of the liver is only in periportal area and very small in amount. Primary lymphoma is easily identified on cross-sectional images; however, secondary lymphoma is sometimes hard to detect because infiltrative, microscopic involvement is not uncommon. At early stage, hepatic lymphomas spread in the periportal area where the majority of the lymphatic tissue is present. Hepatic involvement of Hodgkin’s disease is almost always accompanied by splenic involvement. Hepatic lymphomas can be divided into three groups by their appearance: large solitary masses, multiple nodules, and diffuse infiltrative type. Primary hepatic lymphoma is large solitary type frequently.
On plain radiographs, hepatomegaly or hepatosplenomegaly may be detected. US can visualize the low-echoic, well-defined masses; however, normal liver parenchymal with slightly altered architecture is seen in diffuse form. Multiple small hypoechoic lesions can be mistaken as a diffuse infectious process such as hepatic candidiasis. The most common CT finding is multiple, well-defined, large, homogeneous masses. However, it is very difficult to find area of diffuse infiltration of lymphoma because it may not be distinguishable from normal liver parenchyma. Splenic, renal involvement and multiple lymphadenopathies are accompanying findings at CT scans which can be helpful to make a correct diagnosis of lymphoma. Lymphoma is a poorly vascularized tumor and seen as low attenuated masses after contrast injection. On MRI, lymphomas are visualized as low signal on T1-weighted images and high signal on T2-weighted images. Perilesional enhancement due to lymphoma deposits has been reported.
5.7.2 Plasmacytomas
Extramedullary plasmacytomas usually involve the lungs, paranasal sinuses, or oronasopharynx, and hepatic involvement is rare. Imaging findings of hepatic involvement are nonspecific. On US, target appearance with hypoechoic halo is seen and difficult to be differentiated from that of metastases or lymphoma. The halo is due to the compression of normal peritumoral parenchyma. CT findings are variable: low attenuation without calcifications on unenhanced images and little or no contrast enhancement to moderate enhancement to the level of normal liver parenchyma. Peripheral enhancement and gradual fill-in, like a cavernous hemangioma, were also reported. MRI findings are also nonspecific: high signal on T2-weighted images and low signal in T1-weighted images. On MRI, target appearance was also reported after the injection of contrast agent.
5.8 Summary
1.
Intrahepatic cholangiocarcinomas can be divided into three subtypes depending on their gross morphological features: mass-forming, periductal infiltrative, and intraductal papillary growth type.
2.
Capsular retractions may be observed in cholangiocarcinomas, epithelioid hemangioendotheliomas, and metastases with prominent desmoplastic reaction.
3.
Unusual hepatic sarcomas frequently show large, solitary mass with central necrosis.
4.
MRI with hepatocyte-specific contrast agents is superior to MDCT and PET in detecting small hepatic metastases.
5.
Hepatic lymphomas can be divided into three groups by their appearance: large solitary masses, multiple nodules, and diffuse infiltrative type.
5.9 Illustrations: Other Malignant Tumors of the Liver
5.9.1 Drawing Illustration of Various Types of Cholangiocarcinomas as Classified by the Liver Cancer Study Group of Japan
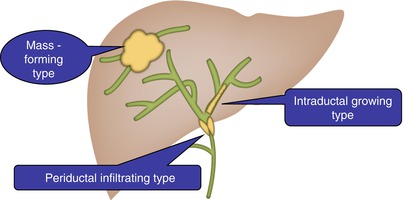
Fig. 5.1
Drawing illustration of various types of cholangiocarcinomas as classified by the Liver Cancer Study Group of Japan. Mass-forming type: discrete, large, and lobulated mass in liver parenchyma. Periductal infiltrative type: tumor infiltration along the wall of bile ducts, resulting in irregular, concentric, and segmental wall thickening and leading to the stricture or obstruction of bile ducts. Dilatation of peripheral bile ducts is commonly observed. Intraductal growth type: papillary tumors limited to the bile duct mucosa. Small polypoid tumors spreading along the lumen of bile ducts. Multiplicity is one of the characteristics of this type
5.9.2 Typical Enhancement Pattern of Mass-Forming-Type Intrahepatic Cholangiocarcinoma
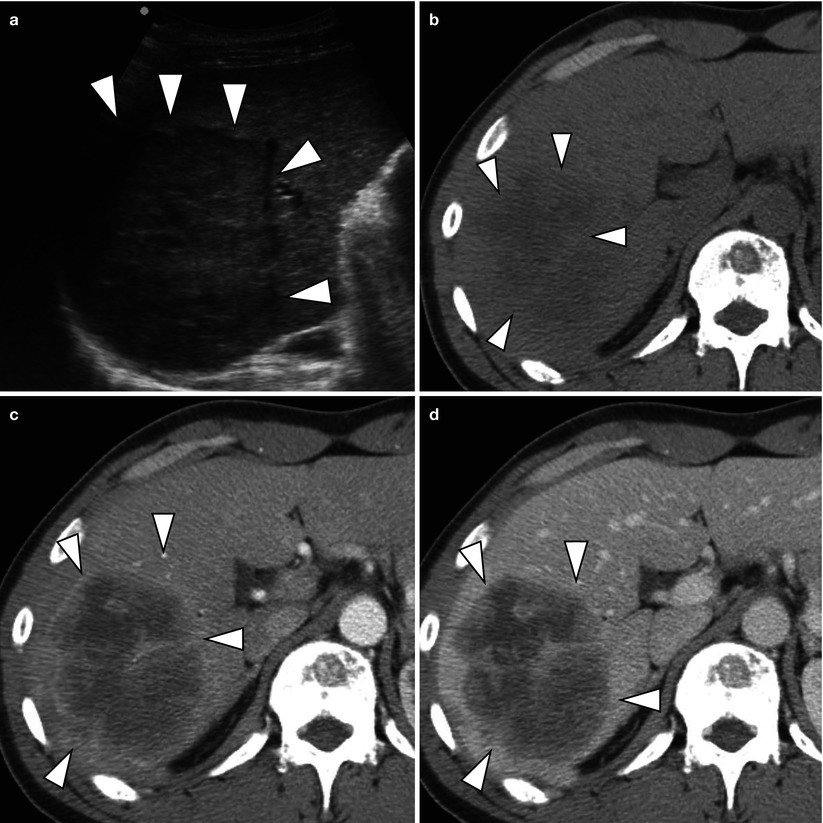
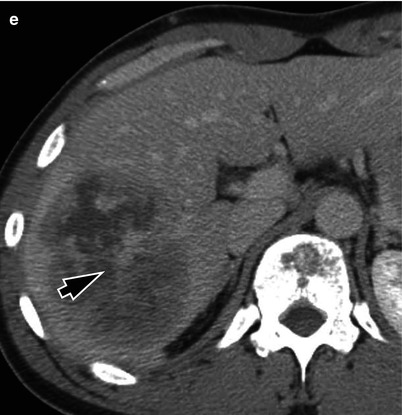
Fig. 5.2
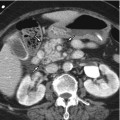
Typical enhancement pattern of mass-forming-type intrahepatic cholangiocarcinoma in a 47-year-old man. (a) US demonstrates a hypoechoic mass with distinctive border (arrowheads). Some heterogeneity of the tumor is noted. (b) Non-contrast CT scan shows a low attenuating mass (arrowheads) in the liver. (c) Arterial phase CT scan demonstrates peripheral, rim-like enhancement of the tumor (arrowheads) with central low attenuating area. (d) Portal venous phase CT scan shows more prominent enhancement of the peripheral portion of the tumor (arrowheads) comparing to the arterial phase CT image. (e) Delayed phase CT scan delineates prolonged enhancement of central portion of the tumor (arrow) due to prominent fibrosis
5.9.3 Typical MRI Findings of Mass-Forming-Type Intrahepatic Cholangiocarcinoma
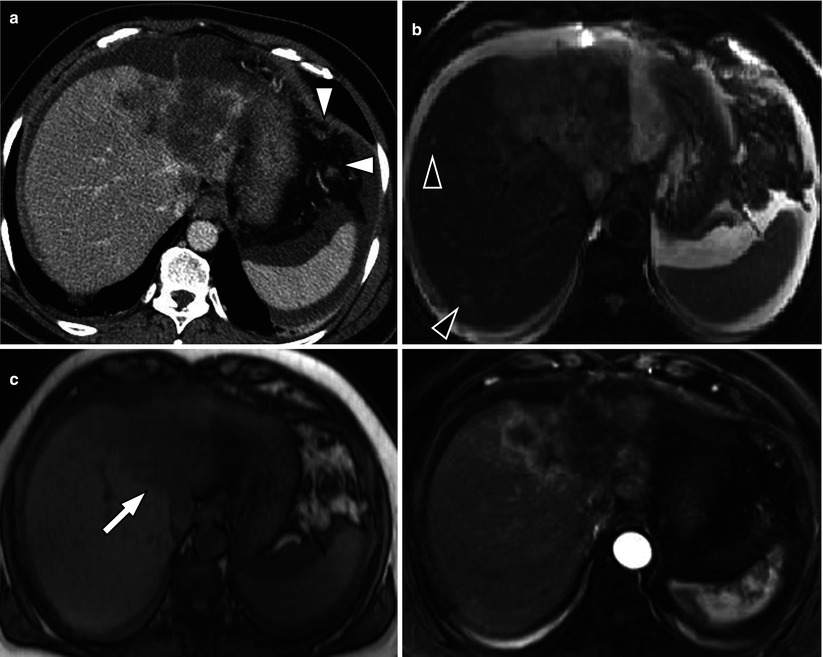
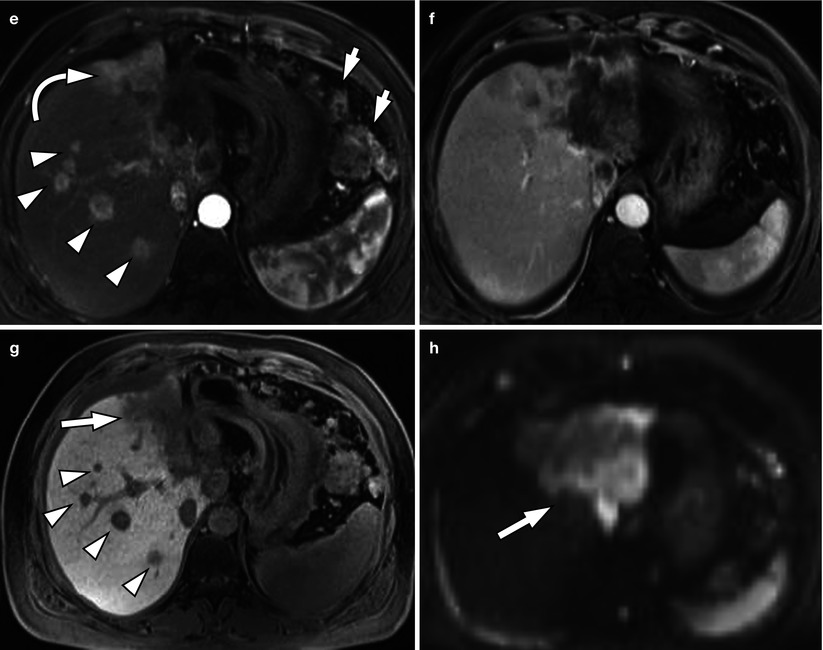
Fig. 5.3
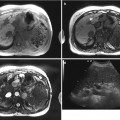
Typical MRI findings of mass-forming-type intrahepatic cholangiocarcinoma in a 62-year-old woman. (a) Portal venous phase CT scan shows low attenuating mass in left hepatic lobe with capsular retraction. Ascites and omental seeding nodules are noted (arrowheads). (b) T2-weighted image demonstrates mass of high signal intensity. Intrahepatic metastases are noted in right hepatic lobe (arrowheads). (c) T1-weighted image shows mass of low signal intensity (arrow). (d) Arterial phase image after injection of Gd-EOB-DTPA delineates peripheral enhancement of the tumor. (e) On another level of arterial phase image, multiple satellite nodules (arrowhead) are enhancing well. Small intrahepatic metastases of cholangiocarcinoma usually enhance well because necrotic area is not common in smaller lesions. Large mass of left hepatic lobe (curved arrow) and conglomerated seeding nodules (arrows) are also noted. (f) On portal venous phase, mass is seen as low signal intensity lesion, and (g) hepatobiliary phase image 20 min after contrast injection shows low-signal defect of the mass (arrow) and multiple intrahepatic metastases (arrowheads). (h) On diffusion-weighted image, mass is seen as a lesion with very high signal intensity (diffusion restriction) compared to the hepatic parenchyma (arrow)
5.9.4 Mass-Forming-Type Intrahepatic Cholangiocarcinoma with Typical Enhancement Pattern and Capsular Retraction
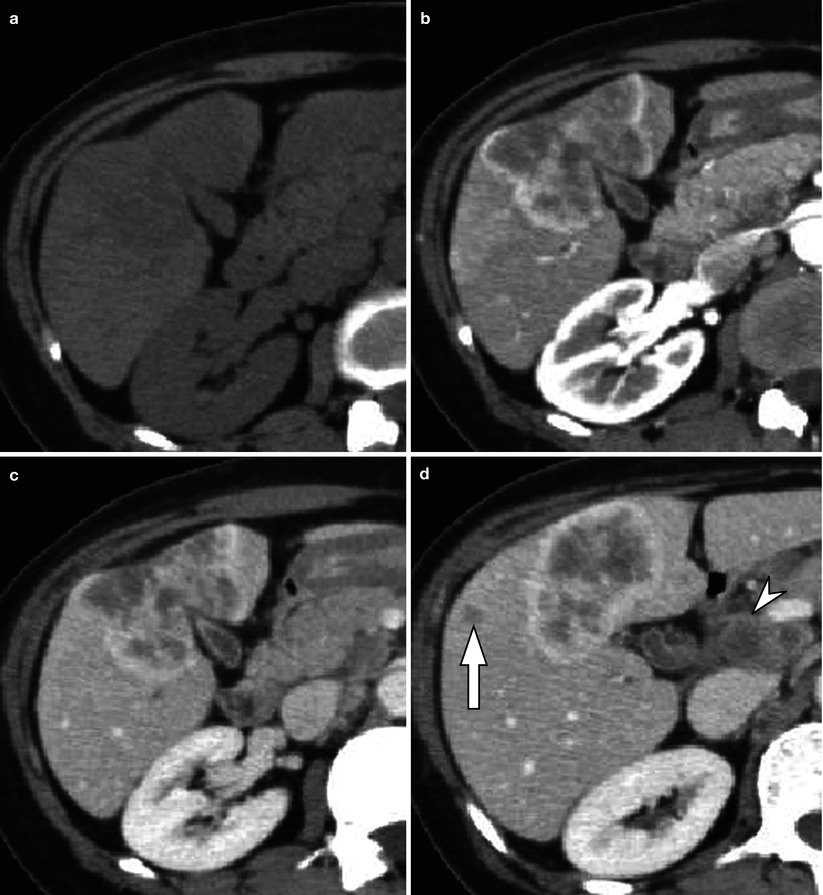
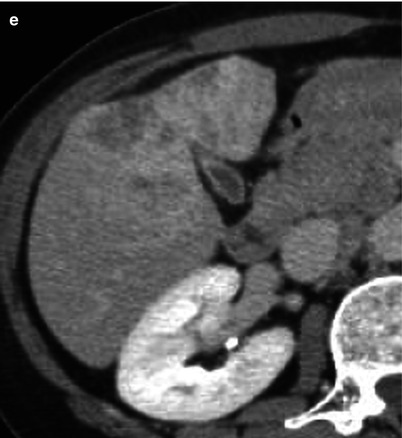
Fig. 5.4
Mass-forming-type intrahepatic cholangiocarcinoma with typical enhancement pattern and capsular retraction in a 55-year-old woman. (a) Non-contrast CT scan shows ill-defined low attenuating lesion mass in left medial segment (S4) and right anterior inferior segment (S5) of the liver. (b) Arterial phase CT scan demonstrates peripheral, rim enhancement of the tumor. Prominent capsular retraction is also noted. (c) Portal venous phase CT scan shows progression of enhancement in central portion of the tumor. (d) On another level of portal venous phase CT scan, metastatic lymphadenopathy at portacaval space (arrowhead) and intrahepatic metastasis (arrow) are delineated. (e) Delayed phase CT scan demonstrates prolonged enhancement of the central portion of the tumor due to fibrosis
5.9.5 Mass-Forming-Type Intrahepatic Cholangiocarcinoma with Diffuse Dilatation of Biliary Tree
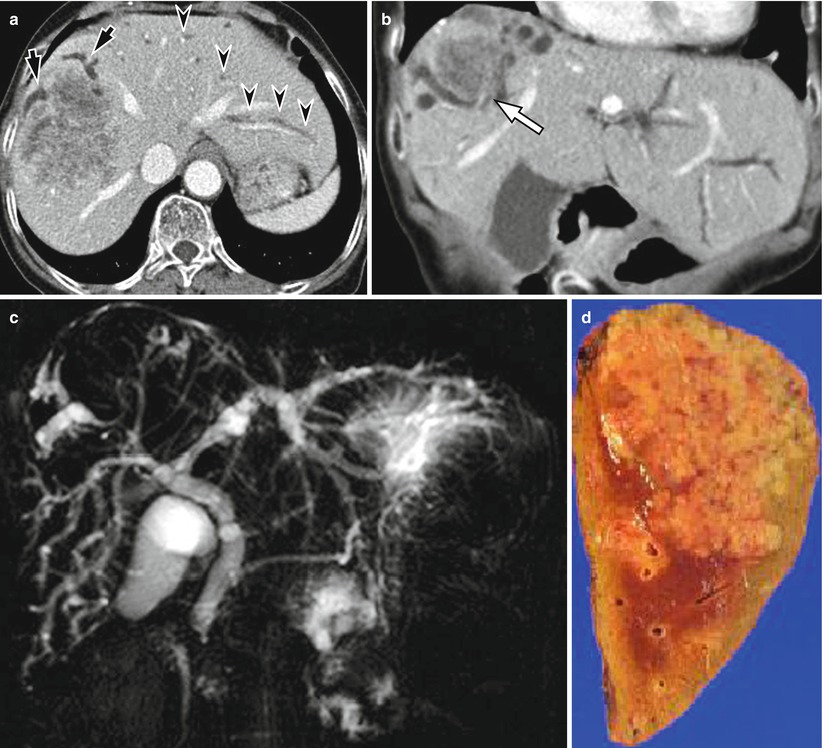
Fig. 5.5
Mass-forming-type intrahepatic cholangiocarcinoma with diffuse dilatation of biliary tree in a 70-year-old woman. (a) On portal venous phase CT scan, there is a heterogeneously enhancing mass in right hepatic lobe. Bile ducts peripheral to the tumor are dilated (arrows). However, bile ducts in other area of the liver are also dilated (arrowheads). (b) Coronal reformatted image shows diffuse dilatation of biliary tree. Mass is located in right hepatic dome (arrow). (c) MR cholangiography shows diffuse dilatation of biliary tree without obstructive lesion in common duct. (d) Cut section of gross specimen delineates whitish tumor with lobulated margin, and this finding is classical “cauliflower appearance”
5.9.6 Small Cholangiocarcinoma Mimicking Hepatocellular Carcinoma
Fig. 5.6
Small cholangiocarcinoma mimicking hepatocellular carcinoma in a 61-year-old woman. (a) On arterial phase CT scan, there is a round enhancing mass in right hepatic dome. Low attenuating rim is noted. (b) Portal venous phase CT scan shows iso- to low attenuating mass with washout pattern. (c) This patient was treated by transarterial chemoembolization (TACE). There is radio-opaque, iodized oil retention (arrow) by TACE. However, after 1 year, progression of the tumor was noted with multiple intrahepatic metastases. Needle biopsy confirmed the diagnosis of cholangiocarcinoma. Small cholangiocarcinomas may enhance homogeneously because there is little necrosis compared to large tumors and can mimic hepatocellular carcinoma
5.9.7 Periductal Infiltrative-Type Intrahepatic Cholangiocarcinoma
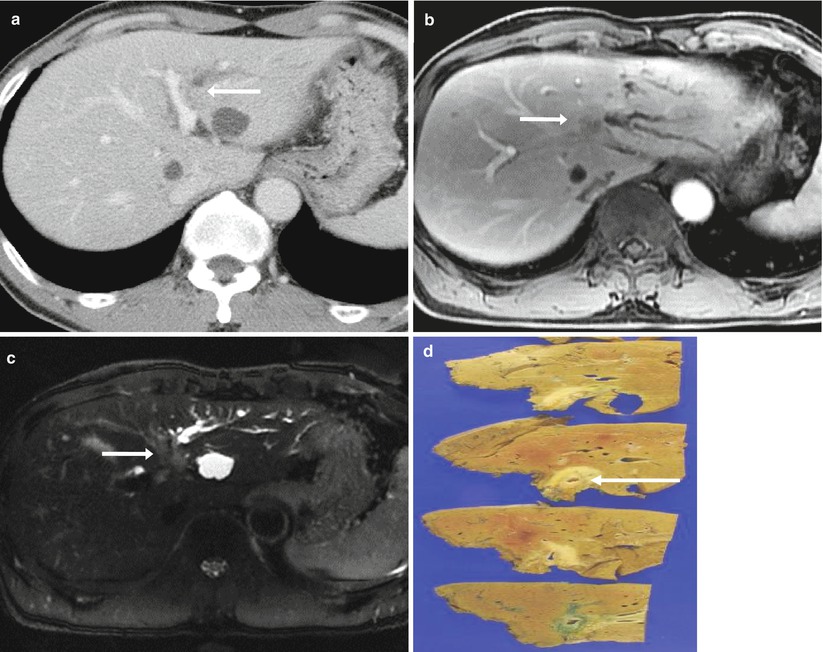
Fig. 5.7
Periductal infiltrative-type intrahepatic cholangiocarcinoma in a 70-year-old man.
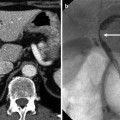
(a) On portal venous phase CT, the tumor is not very clearly displayed. However, isoattenuating lesion obstructs left intrahepatic duct (arrow), and the horizontal segment of left portal vein is indented by the mass.
(b) Portal venous phase MR image shows poorly demarcated soft tissue mass obstructing left hepatic duct.
(c) Axial T2-weighted MR image shows slightly hyperintense mass along the bile duct (arrow).
(d) Photograph of the gross specimen reveals a periductal infiltrating tumor (arrow) along the intrahepatic duct
5.9.8 Intraductal Growth-Type Intrahepatic Cholangiocarcinoma
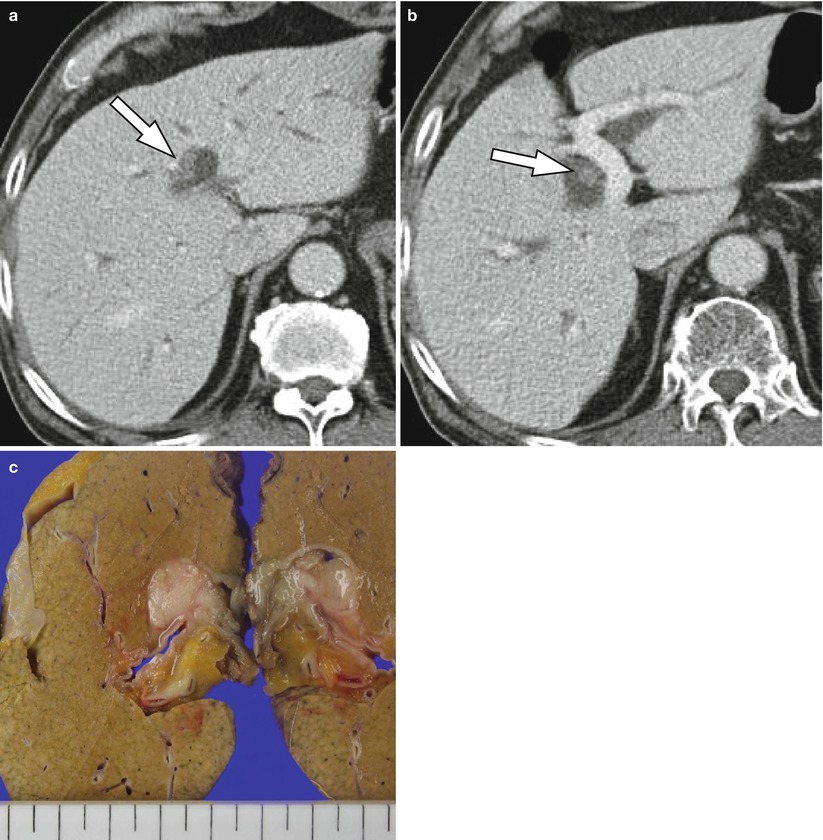
Fig. 5.8
Intraductal growth-type intrahepatic cholangiocarcinoma in a 77-year-old man. (a) Portal venous phase CT image demonstrates higher attenuating intraductal masses (arrow) compared to bile juice. Bile duct dilatation is seen due to mucin production by the tumor. (b) Another level of portal venous phase CT scan shows intraductal lesion (arrow) of same character. (c) On gross specimen, there are polypoid masses filling the lumen of bile ducts (arrowheads)
5.9.9 Intraductal Growth-Type Intrahepatic Cholangiocarcinoma
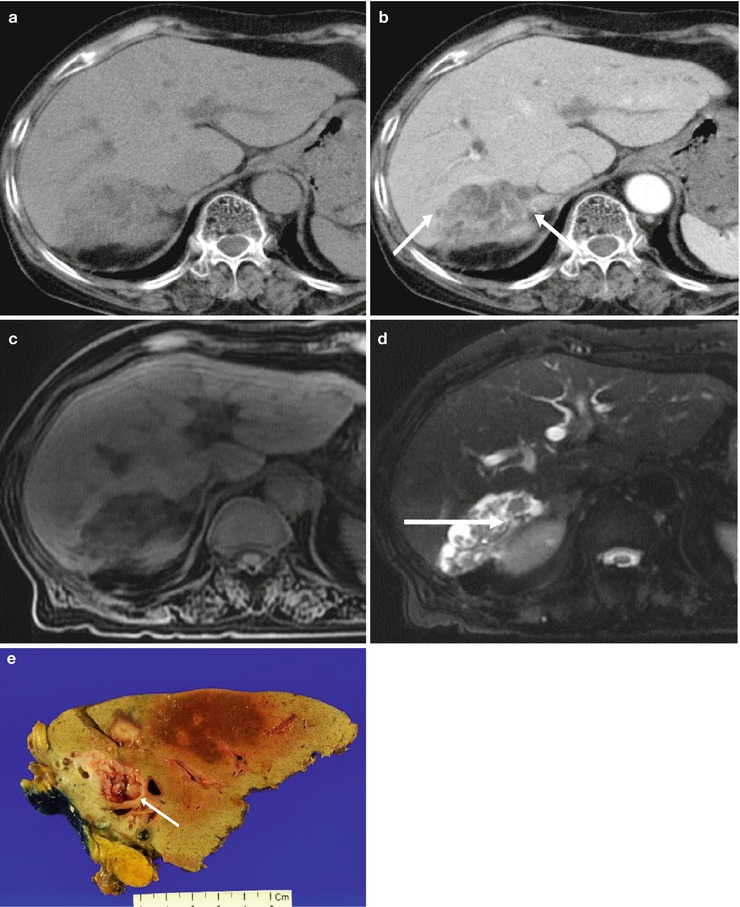
Fig. 5.9
Intrahepatic intraductal-type cholangiocarcinoma in a 75-year-old woman. (a) Non-contrast CT shows a low attenuating mass in right posterior liver. (b) Contrast-enhanced CT during portal venous phase shows intraductal mass within the dilated duct (arrows). (c) T1-weighted MR image shows low signal intensity lesion in right posterior liver. (d) T2-weighted axial MR image reveals intraductal tumor (arrow) and dilated duct clearly. (e) Photograph of the gross specimen shows papillary tumor within the dilated bile duct (arrow)
5.9.10 Intraductal Papillary Mucinous Neoplasm of Bile Ducts
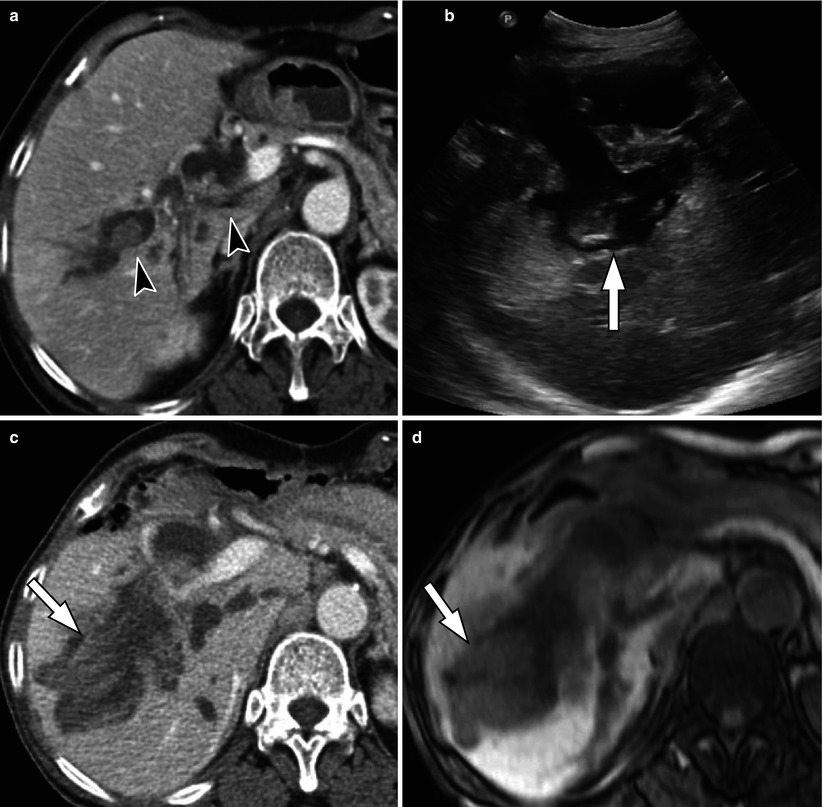
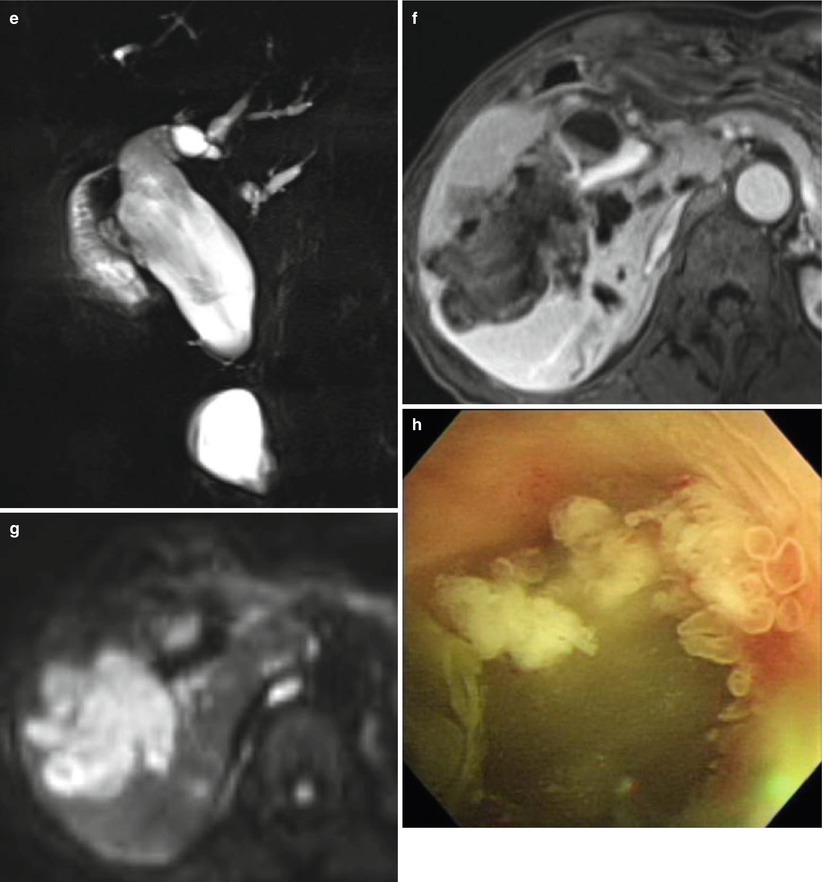
Fig. 5.10
Intraductal papillary mucinous neoplasm of bile ducts in a 69-year-old woman. (a) On portal venous phase CT scan, there are multiple polypoid, intraductal masses (arrowheads), and dilatation of bile ducts is noted. (b) 8 years later, US shows marked dilatation of intrahepatic ducts and polypoid, intraductal mass (arrow). (c) Portal venous phase CT scan shows markedly enlarged intraductal mass (arrow) and prominently dilated bile ducts. (d) On T1-weighted image, this intraductal mass (arrow) is seen as an intermediate signal lesion. (e




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree