This review of the American College of Radiology Ovarian-Adnexal Reporting and Data System (O-RADS) ultrasound (US) v2022 will familiarize the reader with the updated O-RADS US system, highlight new updates, and outline key technical and reporting components. Additionally, this review will outline how to approach and incorporate the system into clinical practice, with reporting and real-world examples. Future directions will focus on addressing knowledge gaps and expanding on research opportunities.
Key points
- •
Ovarian-Adnexal Reporting and Data System (O-RADS) is an evidence-based clinical support system that helps to accurately characterize adnexal and ovarian lesions and predict the risk of malignancy. There are separate versions for ultrasound (US) and magnetic resonance imaging (MRI).
- •
O-RADS US provides suggested follow-up imaging and clinical management, which vary by assessment category.
- •
The O-RADS US v2022 update improves accuracy of the risk stratification and management system and allows the system to be incorporated into all pelvic US reports.
- •
Familiarization with the system, technical components of US examinations, and O-RADS US reporting practices will enable the reader to incorporate the system into their clinical practice.
Introduction
Ultrasound (US) is the imaging test of choice for initial evaluation of ovarian and adnexal pathology. In 2018, the American College of Radiology (ACR) Ovarian-Adnexal Reporting and Data System (O-RADS) US committee published a lexicon with the goal of providing an evidence-based consensus recognized vocabulary for describing normal and abnormal ovarian and adnexal findings. This vocabulary included morphologic descriptors and definitions that help stratify ovarian and adnexal findings based on their sonographic features and assign an associated risk of malignancy (ROM). A subsequent article from the group in 2020 built on this lexicon foundation and outlined risk stratification categories with corresponding ROM based on existing data, as well as management recommendations. , Based on additional information from validation studies and feedback from users, the O-RADS US system was updated (O-RADS US v2022; Box 1 ) to clarify recommendations, incorporate new data, and address clinical challenges. These updates were also incorporated into a smart phone app, which allows O-RADS US to be easily integrated into every day clinical practice.
Major O- RADS US v2022 updates
Governing concepts updates
Expanded applicability criteria to include normal ovaries
Clarified management in patients of uncertain menopausal status and when uterus is absent. Added early and late menopause for management of hemorrhagic cysts
US specialist definition expanded to include importance of experience
Outlined that transabdominal images alone are enough to provide O-RADS US score; transvaginal not required
Interval change assessed with average linear dimension (L + W + H)/3
Clarifies that O-RADS US system can be applied to most lesions regardless of patient symptoms or risk, but that management can be modified. Recommendations are not requirements
Lexicon terms updates
“Bilocular” for cystic lesions
“Shadowing” for solid lesions
Number of locules and lack of internal vascularity added for dermoid cysts, endometriomas, and hemorrhagic cysts
Hyperechoic component of dermoid cysts clarified as to be “diffuse” or “regional”
“Punctate echogenic foci” added as a possible descriptor for endometriomas
“Hydrosalpinx” definition updated to include anechoic fluid-filled tubular structure
Management recommendation updates
Clinical management added for all categories
O-RADS US 0: Repeat US or MRI
O-RADS US 1: No follow-up or additional management
O-RADS US 2: Less follow-up of simple cysts. Surveillance limited to 24 months, previously 5 years; for classic benign lesions, option to follow-up within 3 months if features are suggestive only and overall assessment is uncertain
O-RADS US 3: Follow-up ultrasound within 6 months is now an option; MRI remains an option for solid lesions
O-RADS US 4: Gyn-oncologist imaging protocol now an option
O-RADS US 5: Gyn-oncologist imaging protocol clarified as an option
The O-RADS US v2022 system includes three main components: Governing concepts, O-RADS score with risk assessment, and suggested management. Governing concepts provide insight into how and when to use O-RADS US v2022, clarify definitions, and outline technical considerations ( Fig. 1 ). Users should become familiar with governing concepts before implementing the system into their practice, as they provide rules for system use. The O-RADS US v2022 score is a numeric risk assessment based on lexicon terms, presence and morphology of solid and cystic components, and additional features including lesion size and color Doppler characteristics ( Figs. 2 and 3 ). There are 6 O-RADS US v2022 risk assessment categories (0–5) with increasing associated ROM from 1 to 5 ( Table 1 ). Suggested management is specific for each risk category and has both clinical and imaging components. These recommendations are based on retrospective studies, better align with published expert consensus on adnexal cysts, and represent a unified approach to management best practices based on multidisciplinary panel consensus.



| O-RADS Score | Risk Category | Risk of Malignancy (%) |
|---|---|---|
| 0 | Technically inadequate | N/A |
| 1 | Normal ovary | 0 |
| 2 | Almost certainly benign | <1 |
| 3 | Low risk | 1 to <10 |
| 4 | Intermediate risk | 10 to <50 |
| 5 | High risk | ≥50 |
With this background and framework, the goal of this article is to outline the O-RADS US v2022 system and introduce methods to incorporate it into daily clinical practice.
Imaging techniques
Optimized images are key to both detecting and appropriately diagnosing ovarian or adnexal lesions. Unlike most other imaging modalities, the dynamic nature of US acquisition allows the interpreting physician to have real-time influence on diagnostic quality, and radiologists have a variety of potential methods to improve visualization. Due to this dynamic nature, US also allows for harmonization of imaging and physical examination. Interpretation of US findings should be done in context of patient symptoms, laboratory values, and prior imaging, when available. Complete technical guidance for US examinations has been provided for O-RADS US v2022 and key components will be highlighted here.
Equipment and Documentation
It is recommended that US equipment with transabdominal and transvaginal probes, color Doppler, and cine clip capabilities be used to image the ovaries and adnexa. As a general rule, transvaginal scanning is preferred to transabdominal scanning alone. Equipment maintenance and monitoring should be consistent with the American College of Radiology (ACR)-American Association of Physicists in Medicine (AAPM) Technical Standard for Diagnostic Medical Physics Performance Monitoring of Real Time Ultrasound Equipment. Image acquisition and documentation should follow the established practice parameters for performance of female pelvic US.
Technique
Grayscale and color or power Doppler images of any ovarian or adnexal lesion in orthogonal planes are required. Whenever possible, orthogonal cine clips of a lesion in its entirety are strongly encouraged to ensure that the interpreting radiologist has completely visualized the lesion and can accurately detect its features. Cine clips with color or power Doppler can also be helpful, but are not required, for troubleshooting and confirming findings of internal vascularity identified on static images. The gain, field of view, and focal zone settings should be optimized, and the highest frequency transducer that allows adequate penetration should be used. The size of a detected lesion should be measured in 3 orthogonal planes. Additionally, to differentiate solid components (≥3 mm) from wall irregularity (<3 mm) in cystic lesions, the height of the projection into the cavity should be measured. Images with and without calipers are suggested if calipers could obscure lesion assessment.
Ultrasound Artifacts and Scanning Tips
Acoustic shadowing is a US artifact that results from an increased attenuation of the US beam by a lesion compared to adjacent structures, creating a hypoechoic zone posterior/deep to the lesion. Shadowing is associated with benign dermoid cysts and fibromatous lesions and can be a useful feature to downgrade solid lesions when the outer margins are smooth. It is important to note that shadowing should be broad or diffuse in shape due to attenuation of sound through the structure. Shadowing should not be confused with refractive artifact, which results from sound traveling through adjacent tissues with different acoustic impedances, appearing as linear or focal areas of hypoechogenicity within or posterior to the lesion at the site of the apposed tissue types or reflection of the beam striking the edge of a curved structure.
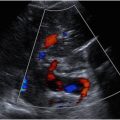
Spatial compounding is a technique used to optimize the appearance of sonographic images by enhancing resolution of tissue planes and reducing noise, but compounding can mask the appearance of posterior acoustic shadowing typically seen with conventional sonography. Turning off spatial compounding, when feasible, may be needed to identify shadowing artifacts. Furthermore, it is important to adjust color Doppler scale to assess for low velocity flow within solid components or septations. This can be accomplished by optimizing the color box to fit the region of interest and decreasing the color scale until a “flash” is achieved and then increasing the scale by one. By optimizing the color scale, it is possible to accurately assess the color score (CS). When flow is equivocal, spectral Doppler can demonstrate a waveform and distinguish flow signal from noise. When flow is minimal, power Doppler can augment sensitivity for slow flow. O-RADS US CS is a subjective assessment of the degree of internal blood flow within a lesion and includes 4 levels: CS 1 (no flow), CS 2 (minimal flow), CS 3 (moderate flow), and CS 4 (very strong flow).
The use of sliding maneuvers, documented with clips, is helpful to confirm lesion location as intraovarian (lesion moves with the ovary when pressure is applied with the transducer) or extraovarian (lesion moves separately from the ovary). These maneuvers can also be helpful for assessing for underlying deep penetrating endometriosis or adhesions. Rapid, gentle probe pressure or “jiggling” can be used to characterize internal cyst contents and determine if internal echoes are mobile or fixed.
How to approach a lesion on ultrasound
The majority of incidentally detected adnexal observations are either physiologic or benign. Thus, the first and most important distinction in O-RADS US is to differentiate physiologic and classic benign findings from cystic or solid lesions, which require further morphologic evaluation and scoring. The O-RADS US system applies an algorithmic approach to assessing lesions with the ultimate goal of increasing the likelihood of correct diagnoses ( Fig. 4 ).

Normal Ovary and Physiologic Cysts
The first major category of observations to consider are physiologic cysts that occur in premenopausal patients, including the follicle and corpus luteum (CL). A follicle is a unilocular, anechoic, cyst with smooth inner walls 3 cm or less; this appearance is considered a simple cyst when greater than 3 cm and in postmenopausal patients. A CL may appear as a thick-walled cyst with smooth or crenulated inner walls. Internally, a CL may be anechoic, have internal echoes (representing blood products) or no apparent cystic center and apposed walls resulting in a solid appearance. Color Doppler is helpful in the diagnosis of a CL, particularly when solid in appearance, by demonstrating peripheral vascularity. While most corpora lutea are 3 cm or less, morphologic features play a greater role in accurate characterization than a discriminatory size cutoff. Physiologic findings are not considered “lesions.”
Classic Benign Lesions
The next major category is the classic benign lesion (CBL) with 6 subtypes (3 intraovarian and 3 extraovarian). These demonstrate typical features, well described in prior literature, without more suspicious characteristics, that allow one to make a specific diagnosis. The most common to arise from the ovary is a hemorrhagic cyst, which is an avascular, unilocular cyst with an internal reticular pattern or retractile clot. Because hemorrhagic cysts generally arise from follicles, they should never occur in late postmenopause (≥5 years of menopause); in this setting, other lexicon terms should be used for cystic or solid lesions. A typical ovarian dermoid cyst has 3 locules or less, no internal vascularity and at least 1 of the following: hyperechoic lines and dots, a diffuse or regional hyperechoic component with shadowing, or floating echogenic spherical structures. A typical endometrioma is an avascular cyst with 3 locules or less and homogenous low-level or ground glass echoes, commonly ovarian in location. An optional feature for endometriomas is the presence of peripheral punctate echogenic foci, which are specific for the diagnosis, though uncommon. Hemorrhagic cysts, dermoid cysts, and endometriomas under 10 cm are O-RADS US 2; if 10 cm or greater, they are O-RADS US 3 (see Fig. 3 ).
The remaining CBLs are adnexal, but extraovarian. First is a paraovarian cyst, which is a simple cyst adjacent to but separate from the ovary and includes paratubal cysts. Second is a peritoneal inclusion cyst, where a loculated fluid collection with or without septations surrounds the ovary. These result from prior surgery or inflammatory process, with scarring and adhesions leading to this accumulation of benign serous peritoneal fluid. The collection should conform to adjacent pelvic organs, and the presence of follicles may help distinguish the ovary from a solid component. Third, a hydrosalpinx is an anechoic, fluid-filled tubular structure separate from the ovary. When seen, incomplete septations (representing folding of the tube) and endosalpingeal folds, which present as equidistantly spaced nodular excrescences along the inner walls, assist in the diagnosis. These 3 classic benign extraovarian lesions receive O-RADS US score 2, and overall size is not relevant for scoring.
While many CBLs will have the typical features described earlier, overall assessment may be uncertain; in this setting, short interval follow-up US may be obtained in 2 to 3 months to allow another opportunity at characterization.
Other Lesions
When an observation does not fulfill criteria for a typical physiologic cyst or CBL, it is placed within the remaining third category, which includes 5 subtypes as follows:
- •
Unilocular cyst without solid components
- •
Unilocular cyst with solid components
- •
Bilocular or multilocular cyst without solid components
- •
Bilocular or multilocular cyst with solid components
- •
Solid lesion
A unilocular cyst has no complete septations, a bilocular cyst has a single smooth, thin, complete septation, and a multilocular cyst has 2 or more septations. Purely cystic lesions are almost never malignant, but risk increases with number of locules and irregular or nodular inner walls and septations. Irregularity of inner walls and/or septations when not a true solid component is defined as focal thickening less than 3 mm in height and is worrisome enough to increase the ROM and the O-RADS US score. If the inner wall is smooth, risk stratification will further depend on maximum size of the lesion (<10 cm vs ≥10 cm), as well as the CS (1–3 vs 4) if multilocular. Management of unilocular and bilocular smooth cysts depends on specified size ranges when less than 10 cm. For unilocular smooth cysts, management will also differ based on the assessment of internal contents as simple or nonsimple defined as internal echoes or incomplete septations.
A solid component may be seen in cystic lesions and is defined as focal wall thickening or solid tissue arising from a cyst wall or septation and protruding into the cyst cavity 3 mm or greater in height, but the largest size of the component is immaterial beyond the 3 mm threshold. Notably, blood products and dermoid contents are not considered solid components in the O-RADS US schema, and denoting them as such will incorrectly upgrade a lesion. Given the most predictive feature for malignancy is solid components followed by complete septations, these two features are the primary focus of the assessment for cystic lesions. A subtype of solid component is the papillary projection that is surrounded by fluid on 3 sides. If a solid component is present, the locularity determines the next step in stratification. For unilocular cysts with solid components, the number of papillary projections (<4 vs ≥4) is relevant for scoring, whereas the degree of internal vascularity (CS 1–2 vs 3–4) is relevant for stratifying bilocular and multilocular cystic lesions with solid components. Overall lesion size is not used for scoring cystic lesions with solid components.
A solid lesion consists of 80% or greater solid component. Assessing the outer contour of a solid lesion is the first step in risk stratification. An irregular or lobular outer contour is worrisome enough to stratify the lesion as an O-RADS US 5 (high risk) lesion without need for further US feature assessment. A smooth outer contour warrants interrogation with color Doppler to determine the CS. When very strong flow (CS 4) is seen, assessment is complete. With less than very strong flow, the presence of broad or diffuse shadowing, commonly seen with fibromatous lesions, is sought. The size of a solid lesion is not relevant for risk assessment.
O-RADS US v2022 assessment categories and management
Using the aforementioned morphologic descriptors, O-RADS US v2022 can be used to stratify ovarian and adnexal lesions into risk assessment categories to help predict the likelihood of malignancy. The following section delineates each O-RADS US category with examples and associated management.
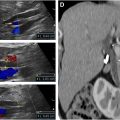
O-RADS US 0: Incomplete due to Technical Factors
O-RADS US 0 is used when an evaluation is incomplete due to technical factors, such as large lesion size, incomplete or nonvisualization, or absence of color or power Doppler images of the lesion ( Fig. 5 ). Repeat US or MRI are both options for additional management based on the clinical scenario and patient factors.