Cerebral vasculopathy in sickle cell anemia (HbSS) is manifest clinically as cerebral infarction and intracranial hemorrhage. The type of stroke, ischemic or hemorrhagic, is age specific with distinct differences in outcomes. Cerebral infarction with or without clinical stroke begins during early childhood and rarely causes death immediately.
Cerebral vasculopathy in sickle cell anemia (HbSS) is manifest clinically as cerebral infarction and intracranial hemorrhage. The type of stroke, ischemic or hemorrhagic, is age specific with distinct differences in outcomes. Cerebral infarction with or without clinical stroke begins during early childhood and rarely causes death immediately . Acute intracranial hemorrhage has been associated with high immediate mortality ranging from 24% to 50% .
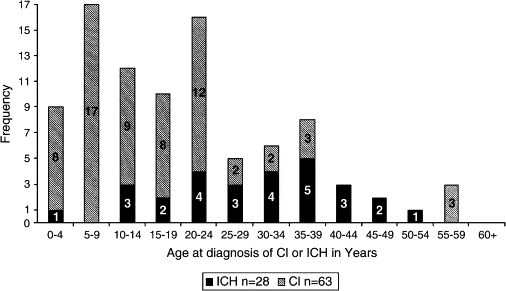
The authors’ overall calculated incidence of first overt infarction in HbSS patients by age 20 years is 11% and by age 45 years 24%. The highest frequency occurs in children aged 2 through 5 years followed by those aged 6 to 9 years. A second peak is observed in adults greater than 20 years of age ( Fig. 1 ). The calculated median age of onset among the authors’ subjects for clinically recognized cerebral infarctions is 13.88 years (range, 1.2 to 58.18) and for intracranial hemorrhage 31.75 years (range, 4.87 to 51.5 years).
The accumulative overall risk for cerebral infarction during the patient’s lifetime, including clinical events and subclinical events, has been estimated to be as high as 30% . This percentage rises even further if one uses improved MRI techniques, particularly fluid-attenuated inversion recovery (FLAIR) . Steen and coworkers reported that 44% of patients demonstrated infarction, ischemia, or atrophy on MRI (FLAIR) and 49% had abnormal findings on MR angiography.
During the last decade, a changing prevalence pattern has been observed, with an increasing frequency of first identified clinical neurologic events at 20 to 25 years of age. Using definitive neuroimaging techniques based on the presence of areas of cerebral atrophy or old infarcts in the border zone regions, it is clear that these patients have “silent” stroke (incomplete infarctions) before overt clinical stroke becomes apparent . The overall prevalence of stroke including cerebral infarction and intracranial hemorrhage is close to reaching the level observed in children between 2 and 9 years of age. The clinical demarcation line is blurring between overt stroke and incomplete infarction, particularly during the third decade of life. This phenomenon is most obvious in the patients who the authors observed during the calendar era of 1980 through 1995 as children. At that time, these patients had evidence of incomplete infarctions based on positron-emission tomography (PET) technology but were not placed on transfusion therapy or hydroxyurea. They now appear with identified neurologic events during the third decade life.
Risk factors
Clinical and laboratory risk factors for cerebral infarction are listed in Box 1 . Seventeen percent of North American HbSS children who had three or more risk factors by age 2 years demonstrated a 38.3% frequency of subsequent clinical stroke by age 8 years . In a prospective study, Glauser and coworkers observed the onset of a first neurologic event between 2.4 and 6.1 years in children who had a transient ischemic attack (TIA) or effervescent neurologic symptoms. These children had incomplete infarction (silent) at a rate of 71% (12 of 17) on conventional MRI. Wang and coworkers showed that 7 of 39 very young neurologically asymptomatic children had significant magnetic resonance neuroimaging abnormalities. Border zone silent infarction combined with elevated blood flow velocity on Doppler analysis increased the risk of overt clinical stroke . Recently, Steen and Ogg have demonstrated that HbSS children have elevated brain N-acetylaspartate, a highly specific marker for neurons. Normal glial cells express a high-affinity for N-acetylaspartate . The transport into glial cells is obligatory for myelination and repair. No risk factor analysis can distinguish between the patient who will progress to clinical stroke and the patient who will sustain slowly enlarging penumbral ischemia or new incomplete infarctions. Border zone infarctions in adults that are identified during childhood seem to be a risk factor for subsequent infarction and intracranial hemorrhage. The observation that the acute chest syndrome rate and recurrence during young childhood is a risk factor for clinical stroke seems to carry over as a comorbid predictor in adult patients with developing chronic pulmonary disease. Systolic hypertension, which is frequently associated with adult onset uremia, is a significant risk factor for intracranial hemorrhage.
Clinical Factors
- •
Age 2 through 8 years (elevated cerebral flood flow)
- •
HbSS sibling with stroke
- •
Incomplete (silent) infarction
- •
Prior TIA
- •
Bacterial meningitis
- •
B19 infection–induced aplastic crisis
- •
Repeat episodes of severe acute chest syndrome with hypoxia (Pa0 2 ) <60 mm Hg
- •
Nocturnal hypoxemia with or without sleep apnea
- •
Acute anemic episode (Hb 2 g/dL below normal level)
- •
Repeat seizure episodes
- •
Dactylitis before age 1 year
- •
Splenic dysfunction or infarction near age 1 year
- •
Priapism
- •
Systolic hypertension a
- •
Decreasing academic school performance
- •
Decreasing fine motor skills (Zurick fine motor examination, Perdue non-dominant hand pegboard)
- •
Abnormal test of variables of attention
Laboratory Risk Factors
- •
Hemoglobin (steady state) concentration <7.5 g/dL with high reticulocyte count
- •
Leukocyte count >15 × 10 9 /L
- •
Platelet count >450 × 10 9 /L
- •
Pocked (pitted) red blood cells ≥3.5% by 24 months of age
- •
Fetal hemoglobin ≤13% by age 24 months
- •
No alpha gene deletion
- •
HLA subtypes (DPBI ∗ 0401)
- •
HLA-A ∗ 0102, HLA-A ∗ 2612
Observations in young children during steady state, not recently transfused, and not on chemotherapy (hydroxyurea) . In a prospective natural history study. For the 17% of North American HbSS children who had three or more risk factors by age 2 years, the frequency of clinical stroke by age 8 years was 38.3% .
a Frequently associated with adult onset uremia and intracranial hemorrhage.
Using the criteria in Box 1 , a high-risk HbSS child should be evaluated at 3 years of age. The neuroimaging evaluation should include ultrasonography of the branches of the internal carotid arteries (ICA), distal carotid siphon (dICA), middle cerebral artery (MCA), and anterior cerebral artery (ACA) ( Fig. 2 ). This evaluation should be followed by diffusion-weighted (DWI) or FLAIR MRI and MR angiography . Abnormalities seen on this combination of studies increase the subsequent infarction risk to greater than 50% in the untreated child . The identification of dICA and MCA stenosis, conventional MRI border zone lesions, and loss of gray matter on quantitative or FLAIR MRI is evidence of disease progression. Cognitive deficiency may be inevitable , although overt stroke and severe cognitive disability should be preventable with adequate treatment .
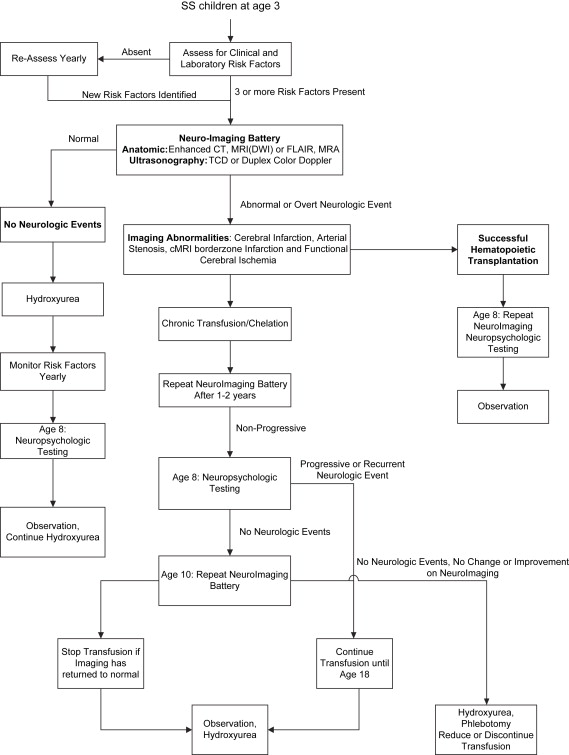
Clinical diagnosis
Clinical diagnosis of cerebral infarction with overt hemiparesis and hemisensory loss is not difficult . More difficult is the diagnosis of less overt dysfunction such as the weakness of one leg or arm. TIAs are not frequently recognized during childhood and adolescence. The reason for this may be the subtleness of the findings and the transient nature of a mild hemiparesis of less than 24 hours. The problem of nonrecognition of mild stroke symptomatology or TIA has been reported in a group of children with and without sickle cell disease. Gabis and coworkers identified that, in children, the time from clinical onset to first medical contact averaged 28.5 hours and the time to diagnosis 35.7 hours. Remarkably, young patients are often not taken to the physician who is aware of the risk of stroke. The patient’s family becomes accustomed to pain in the child’s arms and legs and often states that they thought the weakness of an extremity was secondary to a pain crisis .
The most important aspect of the physical examination at age 3 years during risk assessment is observing the child walking, jumping, standing on one leg, and playing. Any uneven gait or inability to use both hands and arms when placing one block upon another should heighten the sense that this child may already have had an incomplete infarction and should act as a impetus for rapid neurodiagnostic testing .
The association of incomplete (silent) infarction on conventional MRI with an increase in subsequent clinical stroke and cognitive dysfunction has been documented in children and seems to be present in young adults. The progressive accumulation of incomplete neurovascular infarcts leads to poorer intellectual function.
The combined application of anatomic and physiologic neuroimaging modalities in high-risk young HbSS children can improve the predictive accuracy of impending stroke during the first decade of life . Neuroimaging border zone abnormalities without a complete infarction combined with the finding of elevated velocity on ultrasonography define a “window of opportunity” that should prompt the initiation of preventative therapy before the development and diagnosis of overt stroke . Fifty-two percent (52%) of children with border zone abnormalities combined with elevated transcranial Doppler ultrasonography experienced new or expanded silent (incomplete) infarctions or overt stroke. Cognitive disability is a major consequence of microvascular ischemia even without overt clinical stroke . Falling off neurodevelopmental milestones is an indicator for further neuropsychologic evaluation and diagnosis.
Beyond the third decade, intracranial hemorrhage is the most common type of clinical stroke observed. After the third decade of life as end-stage renal disease and chronic lung disease with pulmonary hypertension increase in prevalence, these comorbidities enhance the risk of rupture of small fragile collateral vessels of the brain (Moyamoya like), which are often not recognized clinically to be present.
Clinical diagnosis
Clinical diagnosis of cerebral infarction with overt hemiparesis and hemisensory loss is not difficult . More difficult is the diagnosis of less overt dysfunction such as the weakness of one leg or arm. TIAs are not frequently recognized during childhood and adolescence. The reason for this may be the subtleness of the findings and the transient nature of a mild hemiparesis of less than 24 hours. The problem of nonrecognition of mild stroke symptomatology or TIA has been reported in a group of children with and without sickle cell disease. Gabis and coworkers identified that, in children, the time from clinical onset to first medical contact averaged 28.5 hours and the time to diagnosis 35.7 hours. Remarkably, young patients are often not taken to the physician who is aware of the risk of stroke. The patient’s family becomes accustomed to pain in the child’s arms and legs and often states that they thought the weakness of an extremity was secondary to a pain crisis .
The most important aspect of the physical examination at age 3 years during risk assessment is observing the child walking, jumping, standing on one leg, and playing. Any uneven gait or inability to use both hands and arms when placing one block upon another should heighten the sense that this child may already have had an incomplete infarction and should act as a impetus for rapid neurodiagnostic testing .
The association of incomplete (silent) infarction on conventional MRI with an increase in subsequent clinical stroke and cognitive dysfunction has been documented in children and seems to be present in young adults. The progressive accumulation of incomplete neurovascular infarcts leads to poorer intellectual function.
The combined application of anatomic and physiologic neuroimaging modalities in high-risk young HbSS children can improve the predictive accuracy of impending stroke during the first decade of life . Neuroimaging border zone abnormalities without a complete infarction combined with the finding of elevated velocity on ultrasonography define a “window of opportunity” that should prompt the initiation of preventative therapy before the development and diagnosis of overt stroke . Fifty-two percent (52%) of children with border zone abnormalities combined with elevated transcranial Doppler ultrasonography experienced new or expanded silent (incomplete) infarctions or overt stroke. Cognitive disability is a major consequence of microvascular ischemia even without overt clinical stroke . Falling off neurodevelopmental milestones is an indicator for further neuropsychologic evaluation and diagnosis.
Beyond the third decade, intracranial hemorrhage is the most common type of clinical stroke observed. After the third decade of life as end-stage renal disease and chronic lung disease with pulmonary hypertension increase in prevalence, these comorbidities enhance the risk of rupture of small fragile collateral vessels of the brain (Moyamoya like), which are often not recognized clinically to be present.
Neuroimaging
Essential to the diagnosis of cerebral vasculopathy in HbSS patients is the use of recently developed improvements in CT and MRI . These imaging modalities confirm a clinical diagnosis of cerebral infarction in nearly 100% of HbSS subjects with hemiparetic hemisensory strokes. In addition, enhanced CT and the recently developed techniques of DWI and FLAIR MRI demonstrate exquisite detail about brain structure . Cerebral infarction lesions appear hyperintense on T2-weighted conventional MRI images and are consistently associated with quantitative MRI (DWI) and FLAIR lesions in the contiguous penumbral cerebral lesions. Steen and coworkers reported that FLAIR images demonstrated gray matter abnormalities present in 35% of HbSS subjects without known clinical stroke, similar to findings using PET technology . Kirkham and coworkers noted that perfusion abnormalities on DWI were larger than the conventional MRI infarction regions, identifying penumbral affected regions. FLAIR or DWI abnormalities are associated with soft neurologic symptoms in patients with normal conventional MRI and normal ultrasonography velocities. It is now possible to perform anatomic studies rapidly using DWI or FLAIR images and MR angiography at one time using the same equipment with additional programming. This capability allows for a rapid assessment of the state of the cerebral vasculopathy, defining complete infarctions, incomplete infarctions, early gray matter neuronal loss without overt infarction, and the extent of the affected penumbral regions ( Table 1 ). DWI detects acute ischemia when conventional MRI with spinecho T2-weighted images may still be normal and can be helpful in differentiating acute from chronic ischemic changes. In a small study, acute infarcts less than 6 hours old were detected correctly in only 18% of conventional MRI images, whereas all of the lesions were detected on DWI . Comparison studies have shown equivalent specificity of MR angiography for the diagnosis of cerebral arterial stenosis when compared with conventional angiography or ultrasonography . MR angiography can detect aneurysms of the circle of Willis and the narrow twisted fragile vessels characteristic of Moyamoya anomaly, which are prevalent in patients during the second, third, and fourth decades of life. Programming of T1-weighted MR angiography images can differentiate large vessel endothelial roughening with early stenosis from turbulent blood flow. Turbulence disappears after the anemia is corrected while stenosis of the major vessels remains evident. MR spectroscopy can detect significant metabolic changes in areas of recent ischemia, including the level of N-acetylaspartate. Increases in the level of lactate and glutamate and decreases in the level of N-acetylaspartate can be measured within minutes of the insult and are present for 3 to 6 hours. These acute changes are valuable in the differentiation of old infarction from new ischemic lesions in penumbral regions. In the old infarcts, readily seen on conventional MRI or enhanced CT scans, patients with stable neurologic deficits do not show specific spectral abnormalities.
| Modality | Physiologic activity | Advantages | Disadvantages |
|---|---|---|---|
| Transcranial Doppler ultrasonography (TCD) | Measures elevated blood flow velocity in dICA, MCA, ACA >200 cm/s in anemic children | Portable Best used for screening of presymptomatic cerebrovascular disease | Nonduplex (no direct visualization of vessels) Cannot demonstrate small vessel disease Requires special training Velocity decreases to normal in transfused subjects |
| Modifications of color duplex Doppler ultrasonography including high-resolution B-mode ultrasonography | Measures cerebral blood vessel flow Measures cerebral vessel intima/media thickness >170 cm/s in anemic children is conditionally elevated | Direct duplex visualization of cerebral vessels Good visualization of bifurcation of distal internal carotid artery, posterior cerebral artery, basilar artery Best used for screening for cerebrovascular disease Widely available in tertiary medical centers | In transfused HbSS subjects, velocities decrease to normal range Requires meticulous examination technique |
| Conventional MRI (cMRI) | Acute infarction visible on T1-weighted sequences as low attenuation, hyperintense T2 in completed and border zone incomplete infarction (silent infarction) | Widely available equipment allows high-resolution anatomic visualization of gray and white matter in cerebral infarction Requires modest technical experience | Confinement time in equipment is prolonged; use in unstable patients or during stroke in progression not recommended Poor identification of hemorrhage early |
| Diffusion- and perfusion-weighted MRI (MRI [DWI]) | Identifies molecular displacement of water | Early hyperintense signal detection of acute ischemia before cMRI or CT is sensitive by 8 hours Identifies penumbral areas surrounding infarcts Can be incorporated into routine MRI procedure by adding 3 minutes to immobility time | Requires high-speed echoplanar imaging (EPI) with absolute immobility of head (single shot requires 100 ms) |
| Fluid-attenuated inversion recovery MRI (FLAIR) | Finer definition of neuronal microanatomy Special pulse sequence | Cerebrospinal fluid appears dark White matter lesions, acute infarcts appear bright | Axial sequence is required as a part of the routine brain protocol |
| Quantitative MRI (qMRI) | T1 (spin-lattice relaxation time) demonstrates subtle gray matter abnormalities in subjects with normal cMRI | Very high resolution using available equipment Good correlation with regional cognitive deficiencies | Requires special software and extensive radiologic expertise |
| MR spectroscopy (MRS) | Spectra measures brain lactate, glutamine, N-acetyl aspartate and other metabolites | Rapid onset but short lived elevation of lactate and glutamate with ischemia in gray matter before cMRI or CT abnormalities appear | Adult norms achieved by age 2 years Difficult clinical monitoring during critical period of stroke in progression Subject immobility required |
| MR angiography (MRA) | High-resolution visualization of stenotic carotid and intracerebral arteries | Good correlation to conventional angiography with less adverse risk Uses available MRI equipment with slight increase in time of confinement | Endothelial roughening and irregular blood flow velocity mimics stenosis |
| Computed tomography (CT) | Low-attenuation regions in completed infarctions Cerebral atrophy well defined | Short acquisition time Readily available Acute hemorrhage is hyperintense and identifiable within a few hours of onset | Incomplete (silent) border zone infarctions are often not identified |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree