Score
Color pattern
Stiffness
Histology
1
Green
Homogeneous soft
Normal pancreatic tissue
2
Green, yellow, and red
Soft heterogeneity
Fibrosis
3
Mostly blue with minimal heterogeneity
Hard
Early pancreatic adenocarcinoma
4
Central green hypoechoic region and blue tissue outer layer
Hard
Neuroendocrine tumor, metastasis
5
Blue lesions with heterogeneity due to necrosis
Hard
Advanced pancreatic adenocarcinoma
Giovannini et al. reported in the first study [39] this five-score classification based on different color patterns with a sensitivity of 100 % and a specificity of 67 % in differentiating benign from malignant lesions. Then the same group in the second study [12], basing on the same scoring system, proved that the accuracy was 89.2 % for the differential diagnosis, with both sensitivity and positive predictive value (PPV) over 90 % [34]. A second group, Iglesias-Garcia et al. [13], used a four-score classification (Table 3.2), and they prove that EUS elastography has diagnostic sensitivity, specificity, and overall accuracy of 100 %, 85.5 %, and 94 %, respectively, in diagnosing malignancy [34].
Score | Color pattern | Stiffness | Histology |
|---|---|---|---|
1 | Homogeneous green | Soft | Normal pancreas |
2 | Heterogeneous, green predominant | Soft | Inflammatory pancreatic masses |
3 | Heterogeneous, blue predominant | Hard | Pancreatic malignant tumors |
4 | Homogeneous blue | Hard | Pancreatic neuroendocrine malignant lesions |
Itokawa et al. [11] showed in their study that at qualitative EUS elastography all pancreatic ductal adenocarcinomas had intense blue pattern (hardest color in their color map), whereas inflammatory masses had mixed colorations (green, yellow, and low-intensity blue). Again there are at least two studies that give disappointing results about qualitative endoscopic ultrasound elastography [9, 10, 34]. Hirche et al. [10] found in their study very low values in predicting the nature of pancreatic lesions, such as diagnostic sensitivity, specificity, and accuracy just 41 %, 53 %, and 45 %, respectively. Janssen et al. [9] instead conclude that it is not possible to distinguish chronic pancreatitis from hard malignant tumors with elastography, probably due to their similar fibrous structure. These different values of diagnostic accuracy in qualitative EUS elastography among different studies can be explained probably because of the subjective interpretation of the elastographic pattern [14, 34, 41]. By the way due to the desmoplasia of ductal adenocarcinoma, it is possible to exclude malignancy with high accuracy when in the ROI is seen a predominantly green pattern, so this examination have a negative predictive value usually higher than 90 % [10, 12, 31, 42–44]. With EUS elastography strain ratio (SR) that gives a relative quantitative value, Iglesias-Garcia et al. [40] proved that in differential diagnosis of pancreatic solid lesions quantitative EUS elastography with SR has an accuracy (97.7 %) and a specificity (92.9 %) higher than qualitative analysis and these data could be explained with comparing two regions of interest (ROI), one inside the lesion and one inside adjacent pancreatic parenchyma. They said that a lesion with a SR higher than 6.04 or a mass elasticity lower than 0.05 % has 100 % sensitivity to be classified as a malignant tumor [34, 40]. Furthermore, the specificity can reach 100 % value with a SR higher than 15.41 or a mass elasticity value below 0.03 %. So EUS elastography with SR could be an important tool in the differential diagnosis among pancreatic masses and could differentiate pancreatic cancers from inflammatory masses, with a sensitivity of 100 % and a specificity of 96 %, and pancreatic adenocarcinoma from neuroendocrine tumors, with 100 % sensitivity and 88 % specificity [34, 40]. Another study confirms different values with EUS quantitative techniques with a mean SR of 39.08 (±20.54) for pancreatic ductal adenocarcinoma and a mean SR of 23.66 (±12.65) for inflammatory masses [11, 34]. At the acoustic radiation force impulse (ARFI), being a rigid mass, stiffer than the adjacent pancreatic parenchyma for the pathological features previously described, pancreatic ductal adenocarcinoma is characterized by higher wave velocity values than normal parenchyma with predominately hard pattern at Virtual Touch Tissue Imaging (VTI) and at Virtual Touch Tissue Quantification (VTQ); shear wave velocities are usually >3 m/s, higher than ones measured in the adjacent parenchyma [2, 16, 29, 45]. Park et al. [30] concluded in their study that ARFI elastography identified relative stiffness values between the lesion and background pancreatic parenchyma using VTI and VTQ, which could be helpful in differentiating malignant tumors from benign inflammatory lesions, although there was a considerable degree of overlap between the ARFI values of the benign and malignant lesions. In D’Onofrio et al. experience, a wide range of wave velocity values results from ARFI analysis on pancreatic adenocarcinoma, but in any case a differential diagnosis with chronic pancreatitis is really difficult, and still now no cutoff values exist [2]. Pancreatic ductal adenocarcinoma usually presents at elastography, both qualitative and quantitative, as a harder mass than normal pancreatic adjacent parenchyma; it is also usually harder than benign lesions, but this last feature is not very simple to appreciate at elastographic study, and of course it is harder in relation to the adjacent parenchyma than benign lesions.
3.2.3.2 Other Solid Neoplasms
Neuroendocrine tumors are the second most common pancreatic solid neoplasms, accounting for about 1–2 % of all pancreatic neoplasms. They are epithelial tumors with neuroendocrine differentiation. Most neuroendocrine tumors present as a solitary, solid, well-circumscribed mass with sharp, rounded, or multi-lobulated borders, with an expansive growth pattern compared to the infiltrative one of ductal adenocarcinoma. At pathology neuroendocrine neoplasms typically have an organoid growth pattern, characterized by solid nests. They are tumors with very rich vascularization, responsible of the hypervascular radiological appearance [32, 46–48]. According to studies of Janssen, Hirche, Itokawa, and Giovannini, pancreatic neuroendocrine tumors can appear with a predominantly blue (the hardest color in the map) honeycomb pattern [9–12]; according to Hirche and Iglesias-Garcia, they can appear as homogeneous blue pattern [10, 13]; or according to Itokawa and Giovannini, they can appear as a green central area surrounded by blue tissue [11, 12]. Furthermore, Itokawa and Giovannini [11, 12] demonstrated also a homogeneous green pattern for pancreatic neuroendocrine neoplasms [2]. Iglesias-Garcia et al. [40] proved that endocrine tumors presented the highest strain ratio (mean 52.34) among pancreas focal lesions, leading EUS elastography to be helpful for differentiating pancreatic adenocarcinoma from neuroendocrine tumors with a sensitivity of 100 % and a specificity close to 88 %.
3.2.4 Pancreatic Cystic Neoplasms
In pancreatic cystic lesions study, elastography can have a role, both with strain elastography and with share wave elastography, in particular with ARFI. In literature, there are few studies on cystic lesions and elastography, more about ARFI imaging. The application of ARFI quantification in fluids analysis is absolutely innovative. The basic concept is that different fluids are present into cystic lesions. Even though mechanical waves can propagate through both solid and liquid tissues, instead shear waves are more and highly attenuated in liquids, where only longitudinal waves or the shear wave reflection at the solid–fluid interface can be measured. The large range tissues with different fluid content in vivo, due to different viscosities and the presence of suspended particles, may generate different responses on ARFI imaging [49–51]. Experimental studies on fluid models that showed non-numerical values of share waves velocity at ARFI imaging (XXXX or 0 or X.XX on the monitor) in simple non-viscous fluids such as water have been made [49, 52–54]. The X.XX value can also be registered in solid lesions for several reasons, such as incorrect measurements or technical impairments. On the other hand, the possible result in ARFI fluid analysis is the achievement of a numerical value that can be considered related to the presence of viscous fluid [49–52]. In fluid contents, the obtained values with ARFI are related to the molecular motion inside the region of interest. In simple fluids, such as water, the excessive motion probably determines a too great variation of the individual velocity estimates between tracking beams with the obtaining of an unreliable measurement. In viscous fluids instead the molecular motion is reduced owing to the complex content, with the obtaining of a greater mechanical wave speed [20].
ARFI with the Virtual Touch tissue quantification seems to be able to do a not invasive study of fluid content of pancreatic cystic lesions, potentially improving the lesion characterization, that is nowadays still based on their morphology and architecture at imaging study with an invasive analysis of the fluid content still necessary to make a definitive diagnosis in all doubtful cases [51, 52].
3.2.4.1 Serous Neoplasms
The most common serous neoplasm is the serous cystadenoma, a neoplasm that tends to have a benign behavior. Its typical aspect is characterized by a cluster of microcysts (maximum 2 cm) separated by thin enhancing septa, sometimes forming a central scar, without connection with pancreatic duct. They could also have microcystic, macrocystic, and oligocystic aspects, and, in small percentage, about 5 % of cases, they could resemble a solid lesion due to enhancing septa and absence of visualization of the tiny cysts [20, 55, 56]. As specified by their name, serous cystadenomas are full of simple fluid that could be associated to water as intrinsic features. The value mainly measured in these pancreatic cystic lesions at ARFI quantification imaging is XXXX/0 m/s, as previously explained [20, 49, 52].
In literature microcystic serous cystadenoma aspect at endoscopic elastography was described by Janssen et al. affirming that it appears as a honeycomb predominantly blue pattern [9] and by Hirche et al. reporting that it appears as a homogeneously blue pattern or as a green area surrounded by blue tissue [10].
3.2.4.2 Mucinous Producing Neoplasms
Cystic pancreatic neoplasms producing mucinous are principally mucinous cystadenoma and intraductal papillary mucinous tumor. All these kinds of tumors produce mucus and, as a consequence, are filled by mucina that in this case can be considered a complex fluid. For these reasons, at ARFI quantitative imaging, it is reasonable to expect number values of share waves [49, 52].
3.2.4.3 Differential Diagnosis of Pancreatic Cystic Lesions
Pseudocyst is a complication of acute or chronic pancreatitis. To make diagnosis of pseudocyst of course is very important to know the patient medical history. This kind of pancreatic cyst has a fibrotic wall and is filled by fluid that can be different for hemorrhage or necrosis presence. For these reasons, at ARFI quantification imaging, it is possible to obtain different values of share waves depending on fluid contents, from serous type to very complex one [49, 52].
3.3 Clinical Indications and Guidelines
Transabdominal and endoscopic elastography can sufficiently and reproducibly distinguish between normal pancreatic parenchyma and most pancreatic tumors dominated by stiff (blue) areas. In pancreatic focal solid masses’ characterization, a green predominant pattern is reported to exclude malignancy with high accuracy, whereas a hard or harder than normal pancreatic parenchyma pattern has been reported for tumors [2, 9, 13].
The clinical role of elastographic technique in pancreatic diseases differential diagnosis is however limited, as also showed by Hirche et al. [2, 10]. As a consequence of course, elastography examination cannot substitute the pathologic examination given by FNA or biopsy, so it cannot give a certain pathological diagnosis, but it can be a very useful tool in setting suspicious diagnosis and in guiding diagnostic and treatment management. An important role, giving information about tissue stiffness, can be found for the decision-making process, in particular in those patients with negative or inconclusive FNA in case of very strong clinical suspicious of malignancy. Regarding pancreatic ductal adenocarcinoma, other future applications of elastography could be the tumor stratification based on tissue stiffness predicting differentiation and response to therapy. Moreover a potential pancreatic surgical application of elastography before tumor resection can be the study of pancreatic parenchyma adjacent to the tumor in predicting the risk of postoperative fistula [2, 34].
In the 2013 EFSUMB guidelines about clinical use of ultrasound elastography [57], the role of predominately endoscopic ultrasound elastography is described also, and the recommendations for this study are:
EUS elastography is useful as a complementary tool for the characterization of focal pancreatic lesions.
When there is strong clinical suspicion of pancreatic cancer, but the biopsy is inconclusive or negative, a hard focal lesion on elastography and/or suggestive endoscopic contrast enhancement ultrasound (hypovascular lesion) [58] should guide clinical management by indicating repeat EUS-FNA or direct referral to surgery.
EUS elastography cannot be currently recommended for differentiating advanced chronic pancreatitis from pancreatic carcinoma due to their similar tissue stiffness in a large proportion of cases.
3.4 Image Gallery

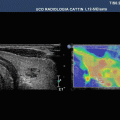
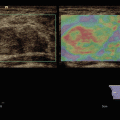
Fig. 3.1
Ductal adenocarcinoma. (a–e) Complete US study: round-shaped hypoechoic mass (calipers in a) located in the pancreatic body with no visible Doppler signals according to the very low vascular density of the neoplastic tissue at color Doppler analysis (b) and resulting stiff (black in c, red in d) due to the rich desmoplastic stroma in the elastogram. At CEUS the lesion typically shows markedly hypovascular pattern (e). (f) Dynamic CT: hypovascular hypodense mass with upstream dilated Wirsung duct

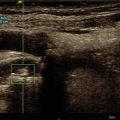
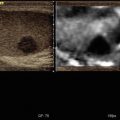
Fig. 3.2
Ductal adenocarcinoma. (a–e) Complete US study: round-shaped hypoechoic mass (a) located in the pancreatic body resulting stiff (marginally red in b, inhomogeneously black in c) due to the rich desmoplastic stroma in the elastogram. At the quantitative ARFI elastography, a velocity value of 3.74 m/s is detected (d). At color Doppler analysis (a), no Doppler signal is visible within the lesion according to the very low vascular density of the neoplastic tissue. At CEUS the lesion typically shows markedly hypovascular pattern (calipers in e). (f) CT study: typical appearance of a pancreatic ductal adenocarcinoma, presenting as a hypovascular lesion (f) in comparison to the adjacent pancreatic parenchyma at venous phase

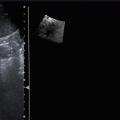
Fig. 3.3
Pancreatic adenocarcinoma. (a–c) MRI study: pancreatic mass hypointense on T1-weighted fat-saturated (a) image. In the pancreatic dynamic phase, the mass is hypovascular (b) with progressive contrast medium retention in the following phases (c). The Wirsung duct is upstream dilated. (d–f) US study: round-shaped hypoechoic mass located in the pancreatic head resulting stiff (slightly black in e) in the elastogram due to the rich desmoplastic stroma. At the quantitative ARFI elastography, a velocity value of 3.54 m/s is detected (f). At CEUS the lesion typically shows markedly hypovascular pattern (calipers in d)

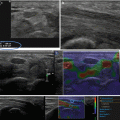
Fig. 3.4
Ductal adenocarcinoma. (a–b) CT study: slightly hypovascular mass in the pancreatic isthmus at pancreatic (a) and venous (b) dynamic phases, causing upstream dilation of the Wirsung duct. (c–f) US study: round-shaped hypoechoic mass (c) located in the pancreas resulting stiff (black in d, red in e) due to the rich desmoplastic stroma in the elastogram. At the quantitative ARFI elastography, a velocity value of 2.14 m/s is detected (f)

Fig. 3.5




Ductal adenocarcinoma. (a–b) CT study: typical appearance of a pancreatic ductal adenocarcinoma, presenting hypovascular in comparison to the adjacent pancreatic parenchyma at pancreatic (a) and venous (b) dynamic phases. (c–f) US study: round-shaped hypoechoic mass (c) located in the pancreas resulting stiff (black in d, red in e) due to the rich desmoplastic stroma in the elastogram. At color Doppler analysis (c), no Doppler signal is visible within the lesion according to the very low vascular density of the neoplastic tissue. At the quantitative ARFI elastography, a velocity value of 1.66 m/s is detected (f)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree