, Gaetano Ciancio1, Alexander Quiroz2 , Alejandro Lugo3 and Javier Casillas4
(1)
Department of Surgery, Miami Transplant Institute, University of Miami Miller School of Medicine, Jackson Memorial Hospital, 1801 Northwest 9th avenue #6, Miami, FL 33136, USA
(2)
Department of Radiology, Jackson Memorial Hospital, 1611 NW 12 Avenue, WW-279, Miami, FL 33136, USA
(3)
Department of Surgery, Miami Transplant Institute, Jackson Memorial Hospital, 1801 NW 9 Avenue #6, Miami, FL 33136, USA
(4)
Department of Radiology, University of Miami Miller School of Medicine, Jackson Memorial Hospital, 1611 NW 12 Avenue, WW-279, Miami, FL 33136, USA
20.2 Introduction
20.3 Indications
20.5 Surgical Procedure
20.5.1 Pancreas Donor Operation
20.5.3 Recipient Operation
20.5.3.1 Vascular Anastomosis (Figs. –)
20.5.3.2 Exocrine Secretions
20.6 Imaging
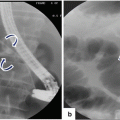
20.6.1 Ultrasound (US) (Figs. –)
20.7.1 Graft Rejection
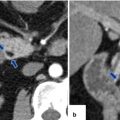
20.7.2 Thrombosis
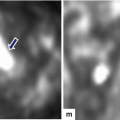
20.7.3 Graft Pancreatitis (Fig. )
20.7.5 Intra-abdominal Abscess
20.9 Teaching Points
20.1 Self-Assessment Questions
1.
Which of the following is the most common type of pancreas transplantation?
a.
Islet cell transplantation
b.
Pancreas transplantation alone
c.
Pancreas after kidney transplant
d.
Simultaneous pancreas-kidney
e.
None of the above
2.
Which of the following is true regarding the surgical technique?
a.
Pancreas allograft is harvested without the duodenum.
b.
Venous drainage into the systemic circulation results in hyper-C-peptidemia.
c.
Y graft consists of the donor’s celiac trunk, and the hepatic and splenic arteries.
d.
Bladder drainage of exocrine secretions is more commonly performed.
e.
Systemic venous drainage is known to have less metabolic complications.
3.
Which of the following is more common in pancreas transplantation than in any other solid organ transplant?
a.
Graft rejection
b.
Graft thrombosis
c.
Recurrent autoimmunity
d.
Anastomotic exocrine leak
e.
Infection
4.
Which of the following is the best tool to diagnose acute graft rejection?
a.
Urinary amylase
b.
Color Doppler ultrasound
c.
Imaging-guided percutaneous biopsy
d.
Unenhanced computed tomography
e.
Magnetic resonance imaging
5.
Which of the following may be improved in patients after a pancreas transplant?
a.
Lipid profile and triglycerides
b.
Left ventricular ejection fraction
c.
Peripheral neuropathy
d.
Retinopathy
e.
All of the above
Answers: 1. d, 2. b, 3. b, 4. c, 5. e
20.2 Introduction
Pancreas transplantation is the optimal therapy for patients with type 1 diabetes mellitus when combined with kidney transplant for end-stage renal failure.
The procedure consists of a whole-organ cadaveric pancreas transplantation with exocrine drainage either to the bladder (Fig. 20.1) or the gastrointestinal tract, using a Y graft for arterial inflow.
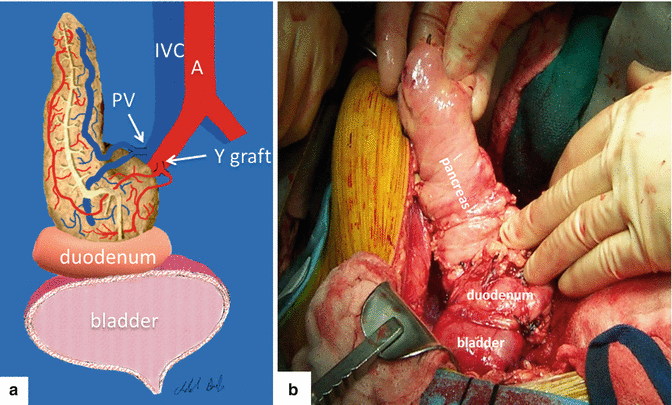
Fig. 20.1
Illustration (a) and intraoperative photograph (b) show the most common type of pancreatic transplantation performed in our institution (bladder exocrine drainage). Aorta (A), inferior vena cava (IVC), portal vein (PV)
The goal of the procedure is to restore normoglycemia by providing sufficient β-cell mass and potentially reduce diabetes-associated complications and improving the patient’s quality of life.
20.3 Indications
Patients with type 1 diabetes (T1D) and risk of secondary complications such as nephropathy, retinopathy, or neuropathy
Diabetic patients with disabling or life-threatening loss of hypoglycemic awareness
20.4 Types of Pancreas Transplants
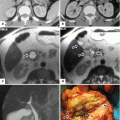
The most common pancreatic transplant is performed in combination with a kidney transplant and accounts for more than 75 % of pancreas transplants (simultaneous pancreas-kidney [SPKT]) (Fig. 20.2).
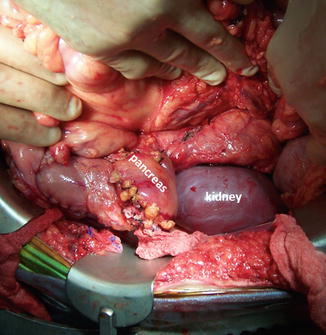
Fig. 20.2
Intraoperative photograph demonstrates a simultaneous pancreas and kidney transplant
The second most common type of pancreas transplantation (16 %) is pancreas after kidney transplantation (PAK).
Pancreas transplantation alone (PTA) is the least common (7 %) and generally has lower graft survival rate.
20.5 Surgical Procedure
20.5.1 Pancreas Donor Operation
The standard donor operation includes procurement of the liver, both kidneys, and the whole pancreas with the duodenum.
Decontamination of the duodenum is performed by means of an antibiotic irrigation (amphotericin B, nystatin, or diluted povidone-iodine) passed through nasogastric tube placed at the level of the pylorus.
The distal donor abdominal aorta is flushed with ice-cold crystalloid followed by either histidine-tryptophan-ketoglutarate (HTK) or Belzer (UW), University of Wisconsin solution.
Once the organs are cooled and the cardiothoracic team has completed the heart/heart-lung procurement, the liver and the pancreas are removed either “en bloc” or separately.
The pancreas, duodenum, and spleen are positioned in a sterile bag with Belzer UW solution, until back table setup is ready.
20.5.2 Back Table Preparation (Figs. 20.3–20.10)
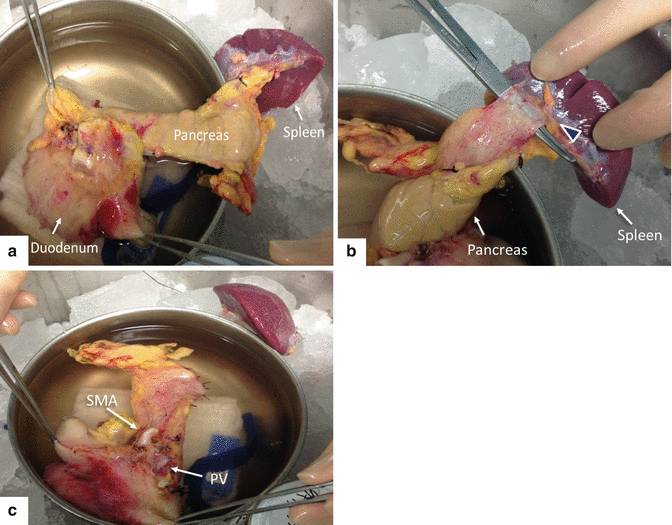
Fig. 20.3
Back table preparation of the pancreatic allograft. Photographs of the pancreatic graft were examined for visible injury, edema, or fat infiltration and show (a) the en bloc graft composed of the spleen, pancreas, and duodenum, (b) dissection of the splenic hilum (arrowhead) with ligation of the splenic vessels, and (c) the completed splenectomy. Note the superior mesenteric artery (SMA) and the portal vein (PV) in the posterior aspect of the pancreatic graft
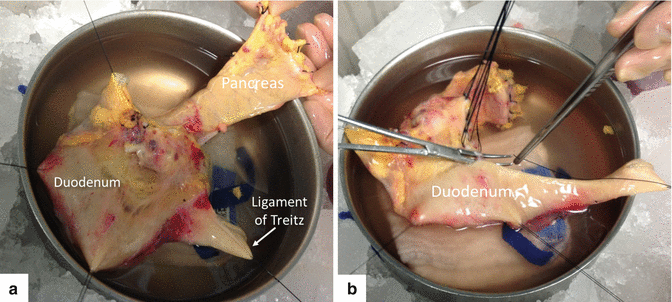
Fig. 20.4
Preparation of the duodenum for implantation. Photographs demonstrate (a) exposure of the duodenum from the distal pylorus to the fourth portion of the duodenum proximal to the ligament of Treitz and the body and tail of the pancreas, (b) dissection of third and fourth portions of the duodenum. This technique is used to create a short duodenal segment for implantation
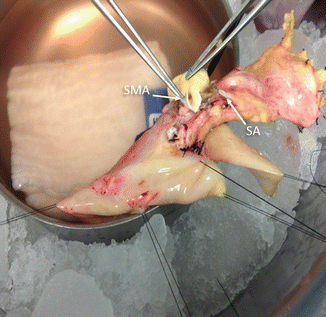
Fig. 20.5
Pancreatic vessels. Photograph shows the transected superior mesenteric artery (SMA) and splenic artery (SA)
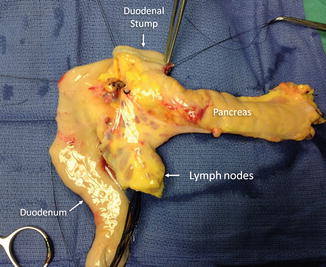
Fig. 20.6
Pancreas during preparation of the duodenal segment. Photograph (anterior view) shows the duodenum with the small bowel mesentery and lymph nodes. Also depicted are the prepyloric duodenal segment (arrow) and pancreas
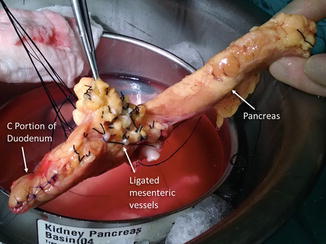
Fig. 20.7
Pancreas with duodenal stump and ligated mesenteric vessels. Photograph shows the entire length of the pancreas with the C portion of the duodenum segment oversewn (arrow) and the ligated mesenteric vessels (arrow)
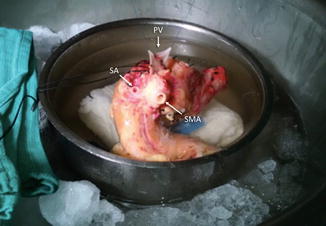
Fig. 20.8
Pancreatic vasculature. Photograph shows the anatomical relationship of the superior mesenteric artery (SMA) (arrow) in relation to the splenic artery (SA) (arrow) and portal vein (PV) (arrow)
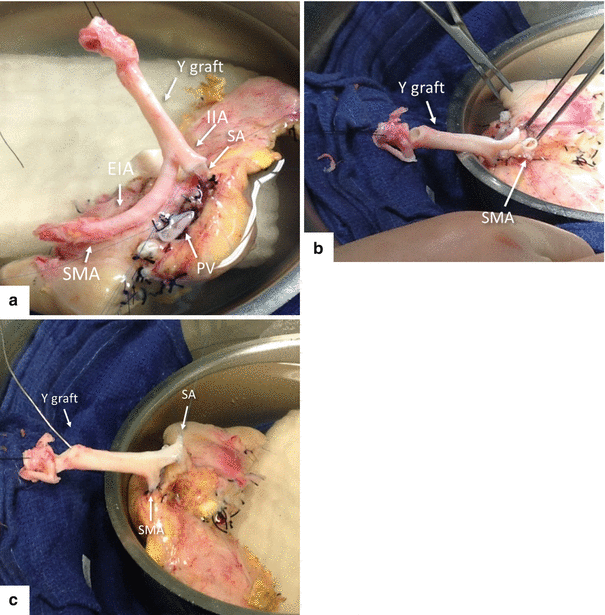
Fig. 20.9
Reconstruction of pancreatic arterial vasculature with Y graft. Photographs (a, b) show the anatomy of the “Y” graft, composed of the common iliac artery and the internal and external iliac arteries. Note the internal iliac already anastomosed to the splenic artery (SA) (arrow) and the external iliac artery (b) being prepared to be anastomosed to the superior mesenteric artery, (c) the “Y” graft anastomosed to the splenic and superior mesenteric arteries
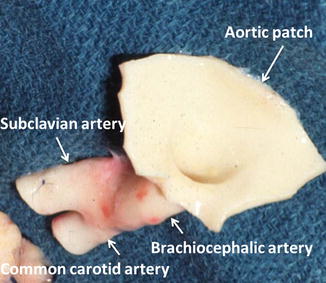
Fig. 20.10
Brachiocephalic trunk, “Y” graft. Photograph shows a brachiocephalic trunk including an aortic patch and the right common carotid and brachiocephalic arteries
The pancreas, duodenum, and spleen are placed in a sterile metal basin containing ice-cold Belzer (UW) solution.
The preparation begins with elimination of fat tissue surrounding the pancreas.
The spleen is removed by ligating the splenic artery and vein.
The duodenal portion of the graft is shortened and only the second portion is kept for use, when bladder drainage is performed.
Both the proximal and distal ends of the duodenum are divided with a stapler and oversewn (Fig. 20.5).
When enteric drainage is performed, a larger length of the duodenum is usually taken, including the third or fourth portion of the duodenum.
The most common technique for arterial reconstruction is to retrieve a naturally bifurcating artery from the donor to be used as arterial “Y” graft.
The most commonly used bifurcation is the common iliac artery into the internal and external iliac arteries which are anastomosed to the splenic artery and superior mesenteric artery, respectively, using 6-0 prolene sutures.
The donor superior mesenteric artery will supply the head of the pancreas and the donor splenic artery will supply the body and tail of the pancreas.
This bifurcated vascular segment is stored in a container of Belzer (UW) solution on the back table.
The portal vein is transected either during the donor operation or at the back table, when the liver and pancreas are taken en bloc at the superior border of the pancreas.
On the back bench, the portal vein is placed on gentle traction with fine sutures and dissected toward the confluence of the superior mesenteric vein (SMV) and splenic vein (SV), ligating and dividing the superior pancreaticoduodenal and coronary veins if present.
Practical Pearls
If the iliac vessels are unfavorable or not usable (trauma or atherosclerosis), the brachiocephalic trunk with the common carotid and subclavian arteries can be used (Fig. 20.11).
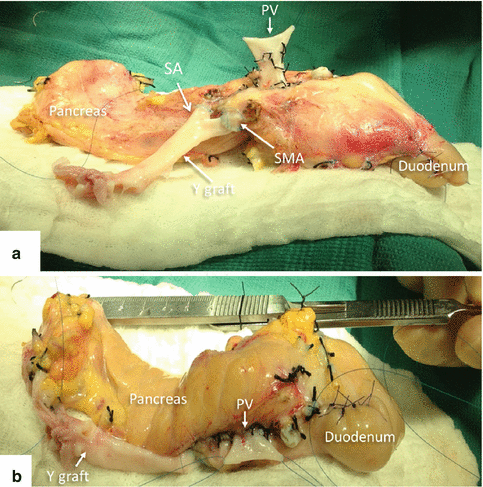
Fig. 20.11
Pancreatic graft with reconstructed vasculature. Photographs (a, b) display the pancreas and the duodenal segment with the Y graft anastomosed to the superior mesenteric artery (SMA), the splenic artery (SA), and the stump of the portal vein (PV). The portal vein will be anastomosed to the common iliac vein for systemic drainage and the Y graft to the common iliac artery
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree