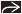Pancreatic Pseudocyst
Brooke R. Jeffrey, MD
Michael P. Federle, MD, FACR
Key Facts
Imaging
Collection of fluid, tissue, debris, pancreatic enzymes, and blood surrounded by fibrous capsule
Develops after 4 weeks of episode of acute pancreatitis
CECT or MR: Enhancement of fibrous pseudocapsule
No enhancement of pseudocyst contents
MRCP: Hyperintense cyst with dilated ducts
Gas within pseudocyst = infection or communication with gut
Pseudoaneurysms can be caused by or simulate a pseudocyst
Top Differential Diagnoses
Mucinous cystic neoplasm
Pancreatic serous cystadenoma
Pancreatic intraductal papillary mucinous tumor
Pathology
Fibrous capsule, no epithelial lining
Clinical Issues
Therapy: Conservative
Spontaneous resolution in 25-40% of patients
Percutaneous drainage
Symptomatic or infected cyst > 4-5 cm
Endoscopic internal drainage: Transduodenal or gastric stents
Surgical therapy: Internal (usually into stomach) or external drainage of cyst
Diagnostic Checklist
Rule out other cystic masses, especially mucinous neoplasms
Often requires aspiration and analysis of cyst contents
TERMINOLOGY
Definitions
Encapsulated peripancreatic fluid collection with fibrous pseudocapsule 4 weeks after episode of acute pancreatitis
IMAGING
General Features
Best diagnostic clue
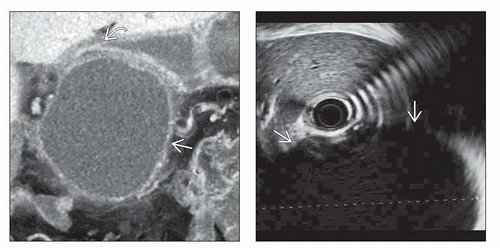
Peripancreatic cystic mass with enhancing pseudocapsule
Location
2/3 located in peripancreatic spaces, including lesser sac and anterior pararenal spaces
1/3
Juxta- or intrasplenic, intrahepatic, psoas compartment, or mediastinum
Size: Varies from 2-10 cm
Morphology
Spherical to oblong
In contrast to true cysts, pseudocysts lack true epithelial lining
Radiographic Findings
ERCP
Communication of pseudocyst with pancreatic duct seen in 70% of cases; decreases over time
CT Findings
NECT
Homogeneous, hypodense lesion with near-water-density (“mature” pseudocyst)
High attenuation indicates blood and gas bubbles indicate infection
CECT
Enhancement of thin fibrous capsule, not of cyst contents
Pseudoaneurysm with arterial attenuation in cyst wall
MR Findings
T1WI
Hypointense; possibly hyperintense (with hemorrhage)
T2WI
Hyperintense (fluid)
Mixed intensity (fluid + debris)
T1WI C+
May show enhancement of fibrous capsule
MRCP
Hyperintense cyst contiguous with dilated pancreatic duct
Ultrasonographic Findings
Grayscale ultrasound
Usually solitary unilocular peripancreatic cystic mass
Multilocular in 6% of cases
Fluid-debris level and internal echoes due to autolysis (blood clot or cellular debris)
Septations (uncommon; sign of infection or hemorrhage or indentation by adjacent artery)
Angiographic Findings
Conventional
For confirmation and embolization of pseudoaneurysm
Splenic artery is most frequently involved, followed by inferior and superior pancreaticoduodenal arteries
Imaging Recommendations
Best imaging tool
CECT or MR, multiplanar
DIFFERENTIAL DIAGNOSIS
Mucinous Cystic Neoplasm
CT or MR: Multiloculated (locules ≤6) hypodense mass
Multilocularity or mural nodules favor tumor over pseudocyst
Often indistinguishable from pseudocyst by imaging alone
More common in women 40-50 years old (“mother lesion”)
Pancreatic Serous Cystadenoma
Benign pancreatic tumor
Most frequently seen in women 50-70 years old (“grandmother lesion”)
CECT
Honeycomb or “sponge” appearance ± central scar
Enhancement of septa delineating small cysts
Unilocular variant indistinguishable from pseudocyst by imaging
Pancreatic Intraductal Papillary Mucinous Neoplasm (IPMN)
Cystic lesion contiguous with dilated MPD sometimes indistinguishable from pseudocyst
Low-grade malignancy arises from MPD > branch pancreatic duct (BPD)
Main duct type causes gross dilatation of MPD ± cystic spaces
Side branch type usually arises in pancreatic head/ uncinate, resembling “cluster of grapes” or small tubular cysts
May be indistinguishable from chronic pancreatitis and pseudocyst
Cystic Neuroendocrine Tumor
Usually noninsulin-producing and nonfunctioning
Tumor: Cystic on NECT and nonenhancing on CECT
No pancreatic ductal dilatation
Diagnosis best by endoscopic US-guided aspiration and biopsy
Congenital Cysts
Associated with von Hippel-Lindau and ADPKD
Rare, usually small and multiple nonenhancing cysts
PATHOLOGY
General Features
Etiology
In 10-20% of patients, acute peripancreatic fluid encapsulates after 4 weeks and forms pseudocyst
Chronic alcoholism (75%)
Abdominal trauma (13%): Major cause in children
Cholelithiasis, pancreatic carcinoma, idiopathic, other causes
Genetics
Genetic predisposition to pancreatitis in some patients even with minimal alcohol intake
Associated abnormalities
Walled-off pancreatic or peripancreatic necrosis
Combination of pancreatic fluid and necrotic debris
Sequelae of acute necrotic collection
Typically forms 4-6 weeks after episode of acute pancreatitis
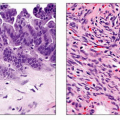
Gross Pathologic & Surgical Features
Peripancreatic fluid collection surrounded by fibrous capsule
Microscopic Features
Absence of epithelial lining
Walls consist of granulation and fibrous tissue
CLINICAL ISSUES
Presentation
Most common signs/symptoms
Clinical significance is related to size and complications
Abdominal pain ± radiation to back (common complaint)
Palpable, tender mass in middle or left upper abdomen
Signs of hemorrhage: Increase in size, bruit over mass, decrease in hemoglobin level and hematocrit
Clinical profile
Patient with history of chronic alcoholism, abdominal pain, and palpable tender mass
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree