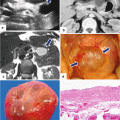
Fig. 16.1
Pancreatic pseudocysts, gross appearance. Intraoperative photograph of a pancreatic pseudocyst (a) and photograph of a gross specimen after Whipple procedure (b) show irregular, bulging cystic masses located in the head of the pancreas. The serosal surfaces of both cystic masses are smooth and glistening with irregular, firm, and fibrous adhesions to the duodenum and adjacent mesentery
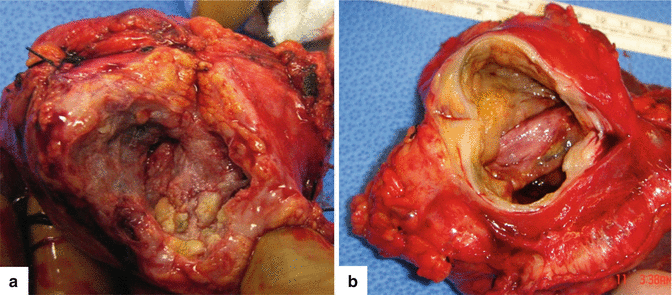
Fig. 16.2
Open section of two pancreatic pseudocysts. Photograph of two longitudinally bivalved cystic masses (a, b) demonstrating unilocular pseudocysts with thick and fibrotic irregular lining surrounding a ragged inner surface
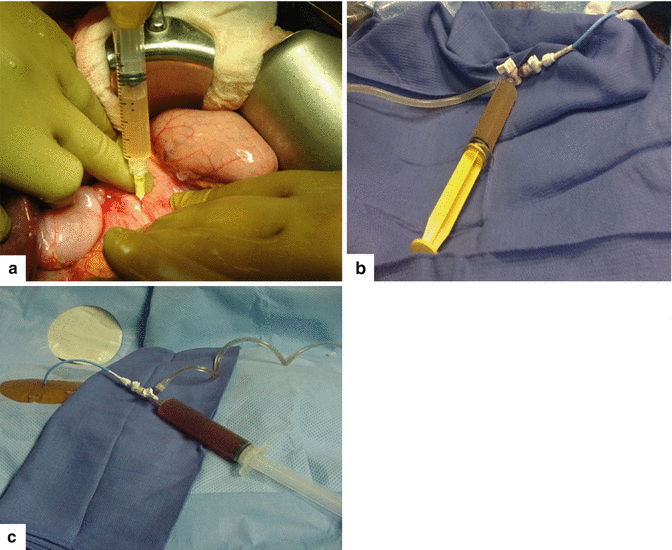
Fig. 16.3
In situ aspiration of three pseudocysts. The fluid content of the pseudocysts can vary in appearance: a clear yellow, transparent serous fluid (a); a turbid and opaque, dusky fluid (b); or a thick brownish fluid (c) with necrotic debris and occasional old blood products. These fluids have high amylase content
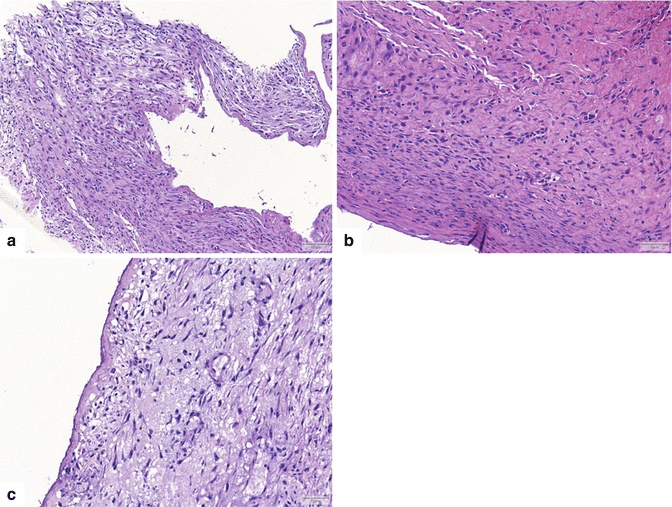
Fig. 16.4
Pancreatic pseudocyst, microscopic appearance. (a) Bands of thick reactive fibroconnective tissue arranged irregularly, creating a cystic cavity (H&E, 20×). (b) Granulation tissue with underlying fibrosis can also be seen (H&E, 10×). (c) At higher magnification, this granulation and fibrous tissue is devoid of epithelium. Prominent chronic inflammation is commonly seen (H&E, 40×)
Cystic mass adherent to adjacent structures (intraoperative)
Cystic mass covered by a thick fibrous capsule
Thick irregular wall and ragged inner surface
Usually contains tissue debris
Fluid: clear yellow, opaque yellow, or brownish (heme pigment)
Microscopic appearance (Fig. 16.4):
Cystic mass lined by fibrous and reactive granulation tissue with macrophages and necrotic debris.
Keynote: pancreatic pseudocysts do not have an epithelial lining.
Practical Pearls
Histologically, the lack of epithelial lining differentiates a pseudocyst from a cystic pancreatic mass.
However, in patients with a long-standing pancreatic cystic neoplasm, the lining can become de-epithelialized making the distinction between a pseudocyst and a true cystic neoplasm difficult. Thus, the pathologist needs to submit the entire cyst for histological evaluation.
16.4 Etiology
Acute pancreatitis (10–20 %)
(Necrosis/liquefaction of the pancreatic or peripancreatic tissue)
Chronic pancreatitis (20–40 %)
(Acute exacerbation or progressive ductal obstruction)
Blunt or penetrating trauma
(Disruption of the pancreatic duct)
Pancreatic surgery
(Disruption of the pancreatic duct)
Practical Pearls
Pseudocysts may occur in patients with pancreatic tumors (adenocarcinoma or IPMN).
The formation of a pseudocyst under these conditions is secondary to the obstruction of the pancreatic duct system by these masses or secondary to the presence of intraductal mucin material.
16.5 Clinical Presentation
Patients with small pseudocysts are usually asymptomatic.
Patients may become symptomatic if the pancreatic pseudocyst:
Expands, gets infected, digests the wall of the adjacent vessels, occludes the adjacent veins, or ruptures into the peritoneal cavity, pleural space, or pericardium
Expansion of the pseudocyst may cause:
Abdominal pain
Vomiting, early satiety (duodenal or gastric obstruction)
Jaundice (biliary obstruction)
Upper GI bleeding (vascular occlusion/thrombosis of the portal vein, splenic vein, or both)
Infection of the pseudocyst may cause:
Fever and elevation of the white blood cell count (WBC)
Sepsis
Digestion of adjacent vessel by the pseudocyst may cause:
Arterial pseudoaneurysm:
Sudden painful expansion of the pseudocyst (bleeding pseudoaneurysm)
Gastrointestinal bleeding, pseudoaneurysm rupture into the pancreatic duct (hemosuccus pancreaticus)
Unexplained drop of the hematocrit (pseudoaneurysm leakage)
Venous compression may cause:
Thrombosis of the splenic, superior mesenteric, or portal vein
Upper GI bleeding
Splenomegaly
Disruption of the pancreatic duct may cause:
Abdominal distention (pancreatic ascites)
Pleural effusion (shortness of breath)
Pericardial effusion (cardiac failure)
16.6 Laboratory Evaluation
The amylase level of the fluid aspirated is usually elevated.
Elevated amylase level alone is not specific to establish the diagnosis (it may also be elevated in IPMNs).
The diagnosis is also supported by the presence of high amylase (above 1,000 IU/L) in pancreatic ascites or pleural effusion.
16.7 Imaging
A unilocular cyst by imaging in a patient with a clinical history of pancreatitis or pancreatic trauma is almost always a pseudocyst.
The diagnosis is further supported by the presence of pancreatic or peripancreatic inflammation, atrophy or calcification of the pancreatic parenchyma, and/or a dilated irregular pancreatic duct with calcifications.
A cystic lesion in the pancreas with no previous history of pancreatitis must be interpreted as a cystic tumor until proven otherwise.
If the diagnosis of pseudocyst is in question, aspiration under endoscopic or conventional ultrasound or CT guidance is recommended.
Ultrasound and computed tomography are the most common modalities used to evaluate pancreatic pseudocyst.
MRI is usually a better diagnostic tool than computed tomography for evaluating pancreatic pseudocysts. Using this method, the cystic nature of the pancreatic lesion and its internal structure can be better displayed. However, the limitations of this imaging modality are the cost and the availability.
16.7.1 Ultrasound (US)
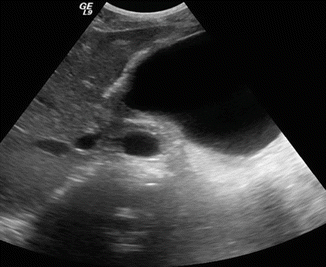
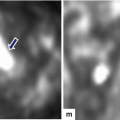
Fig. 16.5
Pancreatic pseudocyst on conventional ultrasound. A 30-year-old female with history of gallstone pancreatitis complaining of epigastric fullness. Transverse image shows a large ovoid cystic mass in the body and tail of the pancreas
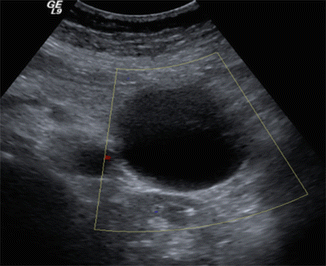
Fig. 16.6
Pancreatic pseudocyst on conventional ultrasound. A 41-year-old male with history of alcohol abuse presenting with abdominal pain radiating to the back. Transverse image demonstrates a round cystic mass in the tail of the pancreas
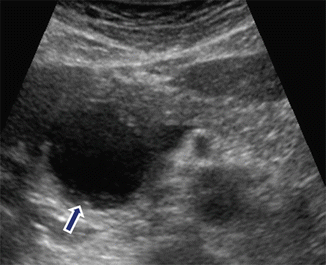
Fig. 16.7
Pancreatic pseudocyst with minimal internal debris on conventional ultrasound. A 32-year-old female with history of drug-related pancreatitis and persistent epigastric pain. Transverse image shows a round cystic mass in the pancreatic head with minimal internal debris in the most dependent portion (arrow)
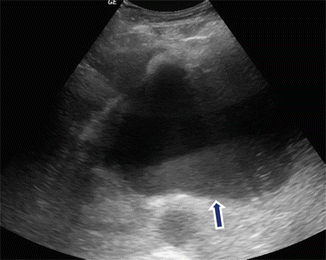
Fig. 16.8
Fluid level of a pancreatic pseudocyst on conventional ultrasound. A 48-year-old female with history of gallstone pancreatitis and early satiety. Transverse image demonstrates a cystic mass with dependent low-level echoes (arrow) in the pancreatic body
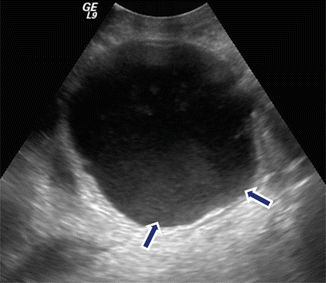
Fig. 16.9
Internal debris inside a pancreatic pseudocyst on conventional ultrasound. A 35-year-old male with history of biliary pancreatitis complaining of mild fullness in the epigastric area. Transverse image of the pancreas shows a complex mass with internal low-level echoes in the dependent portion (arrows)
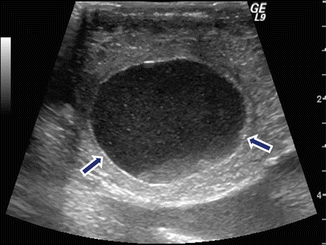
Fig. 16.10
Internal debris of a pancreatic pseudocyst on intraoperative ultrasound. A 43-year-old female patient with history of pancreatitis secondary to alcohol abuse and persistent abdominal pain. Transverse image show an ovoid cystic mass in the head of the pancreas with multiple low-level echoes (debris) (arrows)
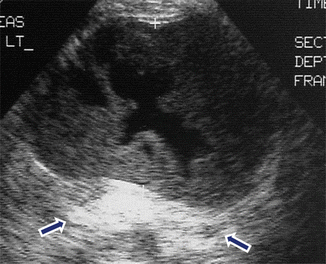
Fig. 16.11
Hemorrhagic pancreatic pseudocyst on conventional ultrasound. A 62-year-old male with history of chronic pancreatitis and abdominal pain. Transverse image of the pancreas demonstrates a complex ovoid mass with an anechoic center, peripheral irregular low-level echoes, and posterior acoustic enhancement (arrows). This cystic mass was aspirated and the fluid revealed high levels of amylase and old blood products
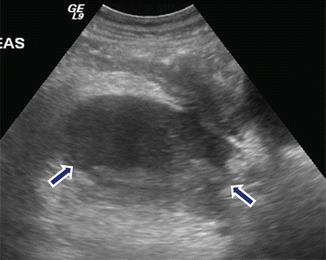
Fig. 16.12
Infected pancreatic pseudocyst on conventional ultrasound. A 52-year-old patient with history of pancreatic pseudocyst presenting with fever and leukocytosis. Transverse image demonstrates a complex ovoid mass in the body and tail of the pancreas with low- and intermediate-level echoes and posterior acoustic enhancement (arrows). Aspiration of this lesion yielded frank purulent material
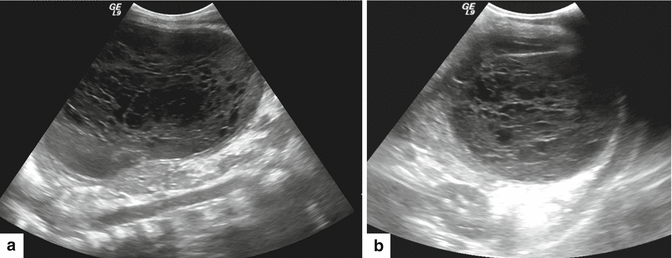
Fig. 16.13
Multiple internal septations in pancreatic pseudocyst on conventional ultrasound. A 3-year-old boy with history of abdominal blunt trauma complaining of abdominal pain. Sagittal (a) and transverse (b) images of the pancreas reveal a large ovoid collection with multiple internal septations. This fluid collection was aspirated and revealed high amylase levels
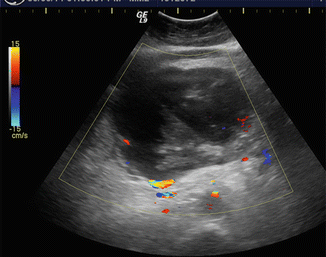
Fig. 16.14
Pancreatic pseudocyst on color Doppler ultrasound. A 61-year-old female patient with biliary pancreatitis. Transverse image of the pancreas shows an avascular ovoid cystic mass with internal echoes in the dependent portion
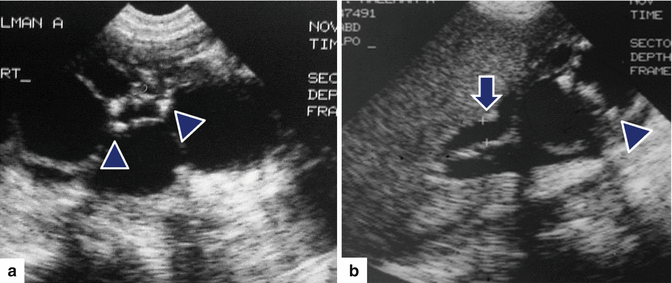
Fig. 16.15
Biliary obstruction secondary to a pancreatic pseudocyst on conventional ultrasound. A 42-year-old male with history of heavy alcohol abuse and chronic pancreatitis. Transverse image (a) demonstrates a multilocular cystic mass in the head of the pancreas. Sagittal image (b) shows dilation of the common bile duct (arrow) secondary to the compression by the multilocular cystic lesion. The bright echoes identified adjacent to this lesion represent pancreatic calcifications (arrowheads)
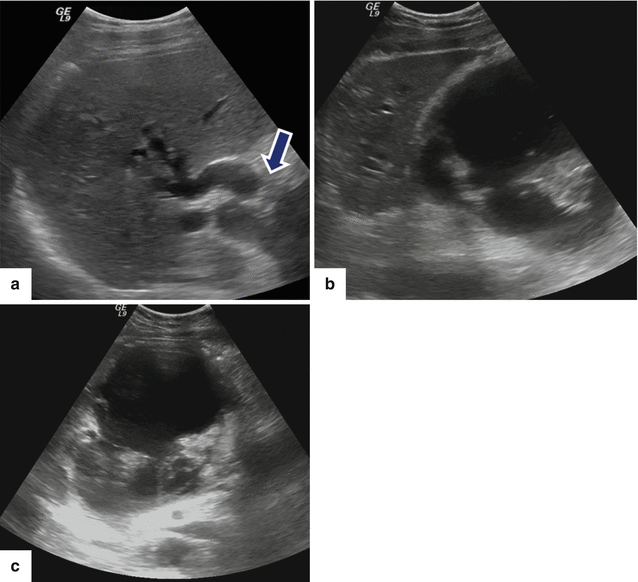
Fig. 16.16
Patient with biliary obstruction secondary to a pancreatic pseudocyst on conventional ultrasound. A 40-year-old male with biliary pancreatitis, abdominal fullness, and jaundice. Sagittal image of the porta hepatis (a) shows dilatation of the intrahepatic and extrahepatic biliary system up to the level of the pancreatic pseudocyst (arrow); sagittal and transverse images (b, c) of the head of the pancreas show a large cystic mass with internal debris
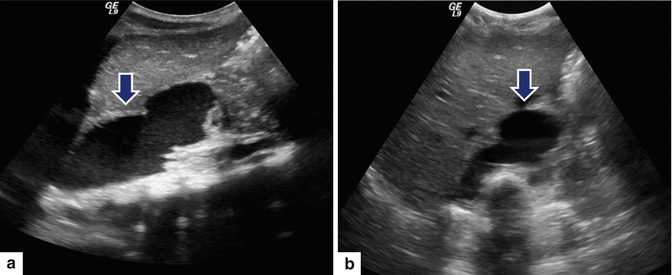
Fig. 16.17
Mediastinal pancreatic pseudocyst on conventional ultrasound. A 9-year-old male with history of previous blunt abdominal trauma and distal pancreatectomy. Sagittal (a) and transverse (b) images of the upper abdomen reveal a large fluid collection with internal debris extending from the neck of the pancreas into the chest cavity (arrows)
Unilocular, avascular, intra- or extrapancreatic, well-circumscribed cystic mass with or without internal low-level echoes (tissue debris)
Intraluminal septations are rare (previous hemorrhage or previous infection)
Associated dilatation of the pancreatic duct and/or calcifications (chronic pancreatitis)
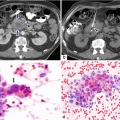
16.7.2 Contrast-Enhanced Computed Tomography (CECT)
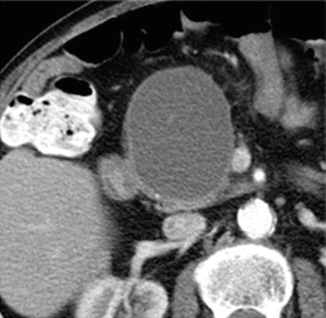
Fig. 16.18
Pancreatic pseudocyst with thin wall on CT. A 41-year-old male with history of gallstone pancreatitis, who came to the emergency room complaining of abdominal discomfort. CECT axial image reveals a large, round cystic mass with a thin, barely perceptible wall in the head of the pancreas
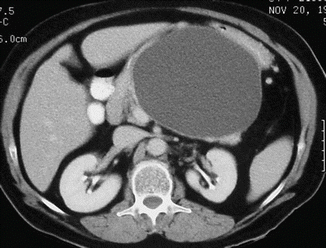
Fig. 16.19
Pancreatic pseudocyst with a thin wall on CT. A 50-year-old male with history of acute pancreatitis. CECT axial image shows a large, ovoid cystic mass with a thin, barely perceptible wall involving the body and tail of the pancreas
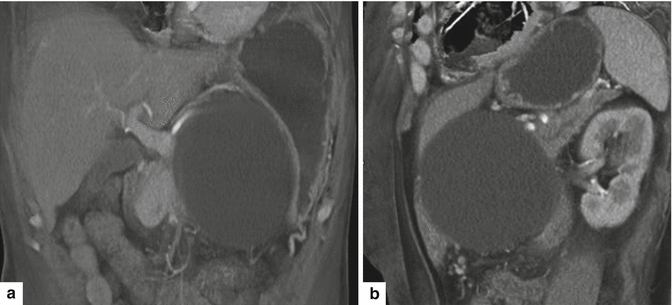
Fig. 16.20
Pancreatic pseudocyst with a thin wall on CT. A 54-year-old female with history of acute pancreatitis secondary to hyperlipidemia complaining of epigastric pain. CECT coronal (a) and oblique (b) images demonstrate a large round cystic mass with a thin, barely perceptible wall involving the head of the pancreas
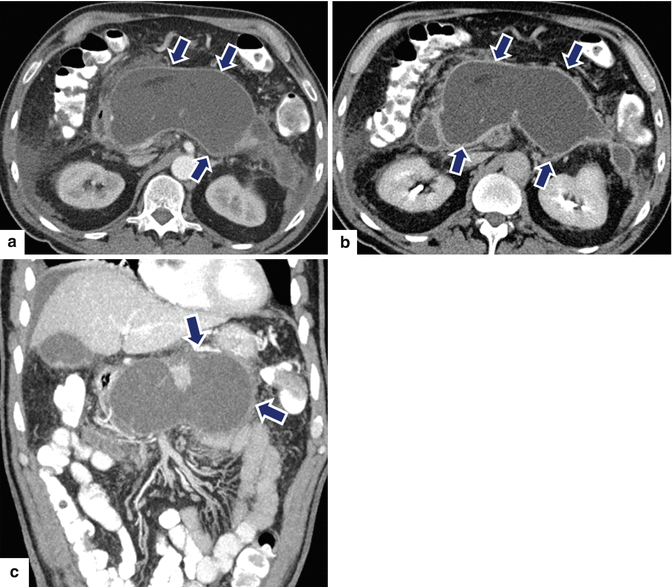
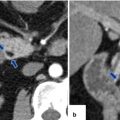
Fig. 16.21
Pancreatic pseudocyst with enhancing wall on CT. A 62-year-old male with dyslipidemia experiencing epigastric pain for 2 days. CECT axial, arterial phase (a), portal phase (b), and axial and coronal plane (c) show a large ovoid cystic mass with an enhancing wall (arrows) involving the body and tail of the pancreas. Note the presence of additional cystic masses in the anterior pararenal space and the peripancreatic inflammatory changes
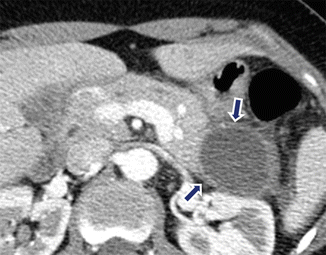
Fig. 16.22
Pancreatic pseudocyst with enhancing wall on CT. A 24-year-old male with alcoholic pancreatitis. CECT demonstrates a cystic mass with a thin enhancing wall (arrows) involving the tail of the pancreas. Note the associated acute peripancreatic inflammatory changes
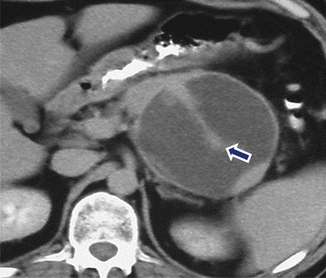
Fig. 16.23
Pancreatic pseudocyst with internal septation on CT. A 31-year-old female with history of gallstone pancreatitis and upper abdominal fullness. CECT shows a round cystic mass with a central thick septum (arrow) in the body and tail of the pancreas
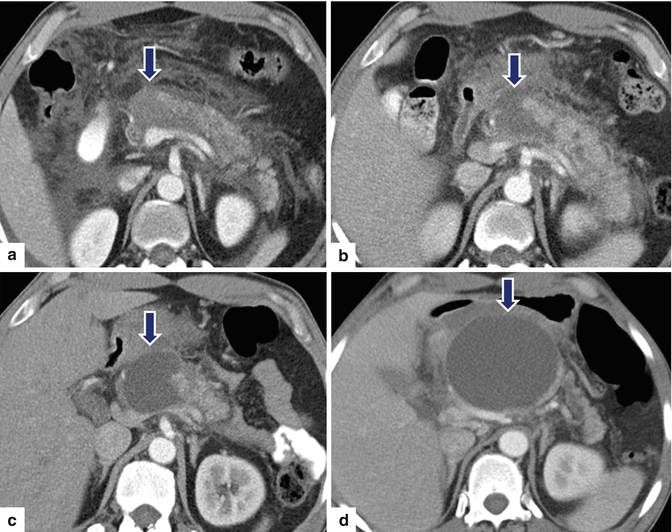
Fig. 16.24
Pancreatic pseudocysts associated with pancreatic duct disruption on CT. A 26-year-old male with history of alcohol abuse was admitted with severe epigastric pain. CECT axial image (a) performed at admission shows peripancreatic inflammatory changes and an area of low attenuation in the neck of the pancreas (arrow). CECT axial image (b) performed 2 weeks later demonstrates that the area of low attenuation in the head of the pancreas appears more conspicuous (arrow). Follow-up CECT performed 3 weeks later (c) shows a fluid collection in the neck of the pancreas (arrows). CECT image (d) taken 6 weeks later shows interval increase of the fluid collection in the neck of the pancreas (arrows). Note how the fluid collection now appears round and well-circumscribed and how the peripancreatic inflammatory changes have resolved
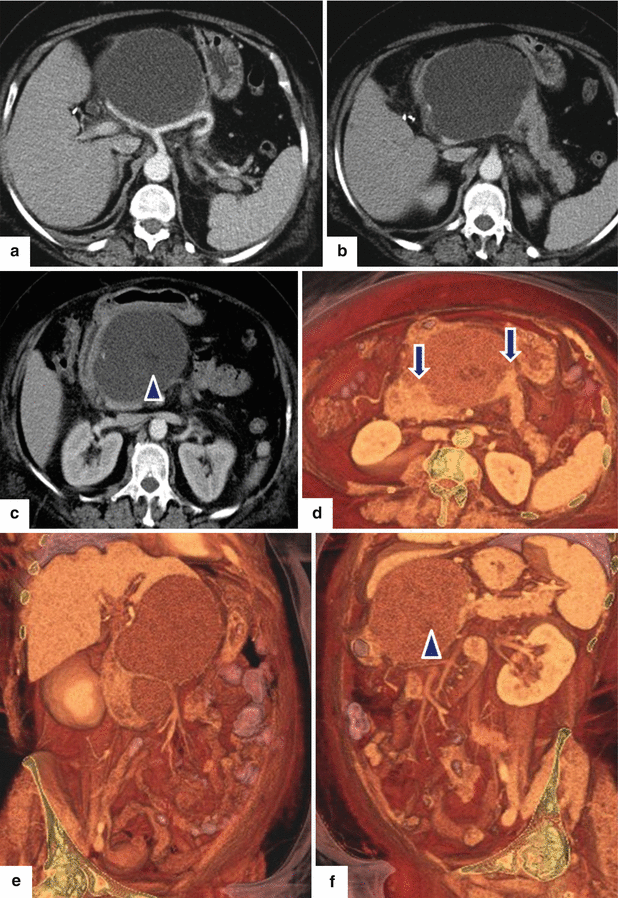
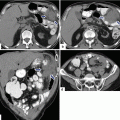
Fig. 16.25
Pancreatic pseudocyst associated with pancreatic duct disruption on CT. A 61-year-old female with biliary pancreatitis complaining of epigastric pain and early satiety. CECT axial images (a–c) and 3D volume-rendered axial (d), coronal (e), and oblique (c) images demonstrate a large septated fluid collection in the neck and proximal body of the pancreas compressing the gastric antrum and duodenum. Note the gap of pancreatic tissue between the proximal and distal pancreas (arrows in d). This collection displaces the hepatic and splenic arteries and contains small areas of low attenuation (fat) (f) (arrowhead)
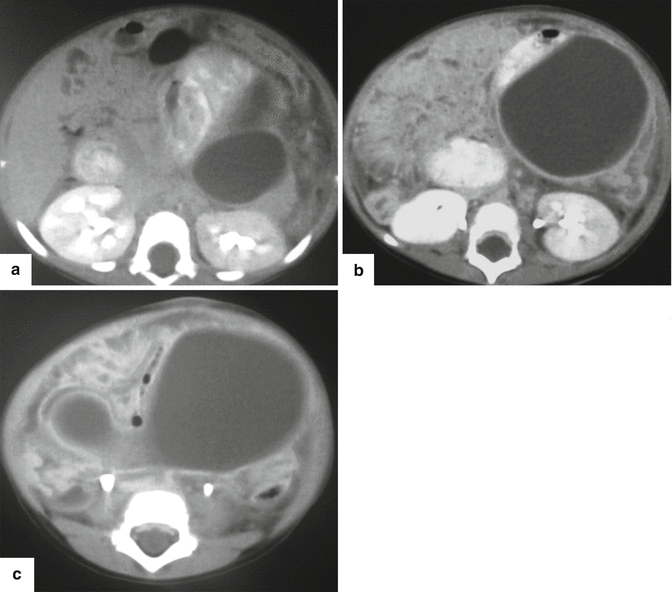
Fig. 16.26
Posttraumatic pancreatic pseudocyst on CT. A 2-year-old male patient with history of child abuse complaining of severe abdominal pain. CECT (a–c) shows a large, round, fluid collection posterior to the stomach and extending inferiorly to the lower abdomen. This collection was drained percutaneously. There was a high content of amylase in the aspirated fluid
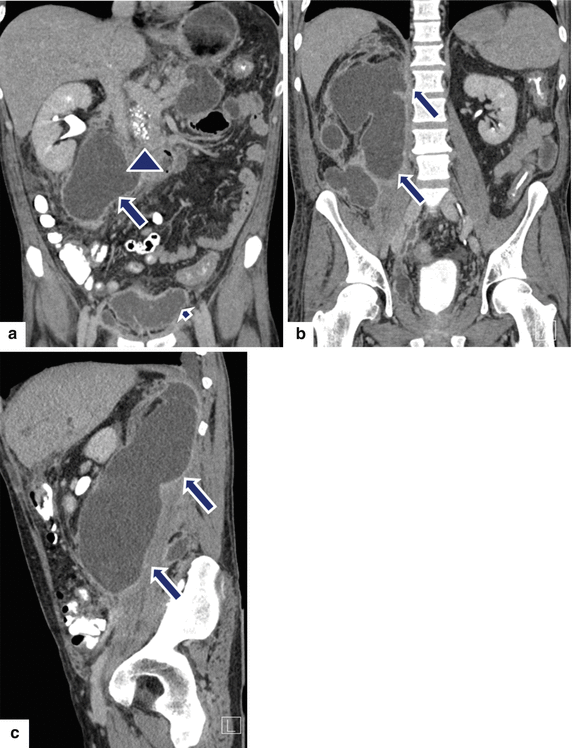
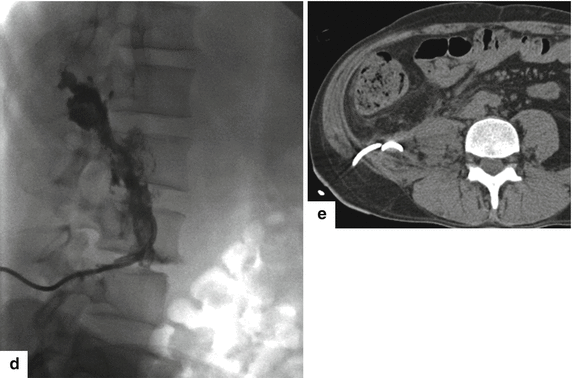
Fig. 16.27
Large extrapancreatic pseudocyst on CT. A 40-year-old male with history of chronic pancreatitis and liver transplant secondary to alcoholic liver cirrhosis 7 years earlier. The patient has been complaining of abdominal pain and weight loss of close to 40 lb. Laboratory: serum amylase of 1,628 units/L and serum lipase 2,912 units/L. CECT coronal (a, b) and sagittal (c) planes demonstrate a large cystic collection in the right posterior pararenal space (arrows) extending into the pelvis and the Retzius space (a) (short arrow). In addition, note the presence of multiple pseudocysts in the retroperitoneum. Multiple calcifications are seen in the pancreas (arrowhead). The large pseudocyst in the retroperitoneum was drained successfully with a percutaneous catheter placed under CT guidance. Contrast injection into the pseudocyst with fluoroscopic guidance (d) shows a large cavity in the retroperitoneum. NCECT axial image (e) obtained 3 weeks later shows complete resolution of the large retroperitoneal pseudocyst
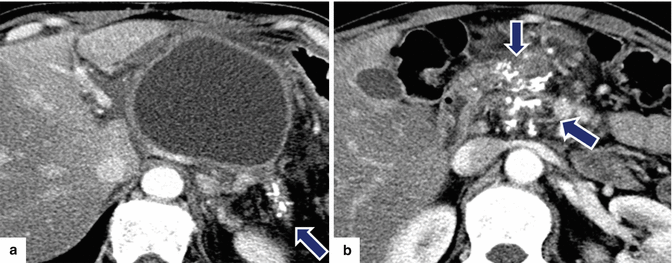
Fig. 16.28
Pancreatic pseudocyst in a patient with chronic pancreatitis on CT. A 48-year-old male with chronic pancreatitis secondary to alcohol abuse complaining of abdominal pain, nausea, and excessive burping. CECT axial images (a, b) reveal a large cystic mass with thick walls in the body and tail of the pancreas. Note the presence of multiple calcifications in the tail and head of the pancreas (arrows)
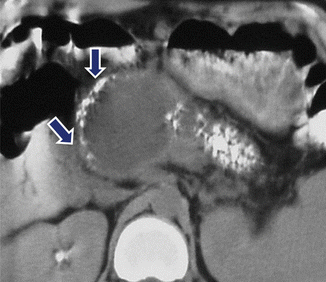
Fig. 16.29
Pancreatic pseudocyst associated with chronic calcified pancreatitis on CT. A 45-year-old woman with history of chronic pancreatitis and epigastric pain. CECT reveals a round cystic mass in the head of the pancreas displacing multiple calcifications (arrows). Calcifications are also identified in the body and tail of the pancreas
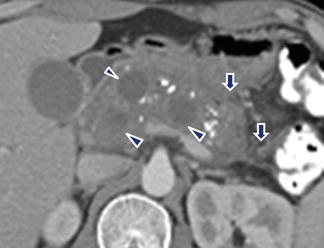
Fig. 16.30
Multiple pancreatic pseudocysts on CT. A 42-year-old male with chronic pancreatitis. CECT axial plane shows multiple, round, cystic masses in the pancreas intercalated with multiple calcifications. Note the inflammatory changes around the pancreas (arrows)
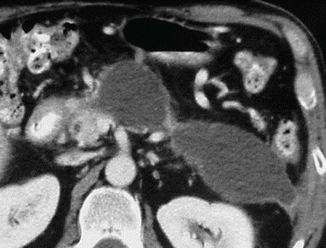
Fig. 16.31
Peripancreatic pseudocysts on CT. A 37-year-old female patient with history of chronic pancreatitis. CECT axial image demonstrates two cystic masses in the left anterior pararenal space
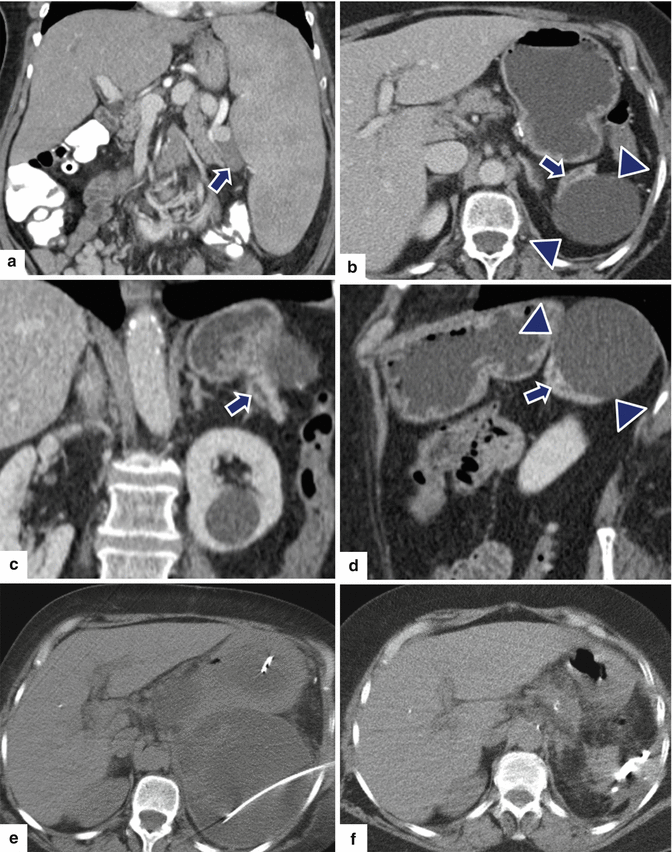
Fig. 16.32




Pancreatic pseudocyst secondary to residual pancreas on CT. A 74-year-old female with splenomegaly and multiple, enlarged, splenic artery lymph nodes secondary to involvement by marginal zone lymphoma. The patient underwent splenectomy with distal pancreatectomy. Two weeks after this procedure the patient returned to the ER with left upper quadrant pain. Preoperative CECT coronal image (a) shows a prominent spleen and enlarged nodes in the splenic hilum (arrow). At the time of readmission, CECT axial (b) and coronal (c, d) images show a large cystic collection in the left upper quadrant (arrowheads). Note a residual fragment of the distal pancreas with a dilated duct seen adjacent to this collection (arrows). This collection was drained percutaneously with CT guidance (e). The fluid drained showed elevated amylase. NCECT (f) obtained 3 weeks later shows complete resolution of the pancreatic pseudocyst
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree