Primary hyperparathyroidism (PHPT) is a common endocrine abnormality, caused in most cases by a single parathyroid adenoma. Surgery remains the first-line curative therapy in PHPT. Imaging plays a vital role in presurgical localization of parathyroid adenomas. Ultrasound provides a safe and quick imaging modality free of ionizing radiation, but is operator dependent. Sestamibi scan offers comparable sensitivity to ultrasound, improved with concurrent tomographic imaging. 4DCT remains a problem-solving technique in challenging cases and after failed neck exploration. We present an overview of various parathyroid imaging modalities, including protocols and findings, in addition to relevant pearls and pitfalls.
Key points
- •
In patients with primary hyperparathyroidism, imaging of parathyroid glands plays an important role in localization of parathyroid adenomas prior to minimally invasive parathyroid resection.
- •
PHPT is caused by a single adenoma in 80% of cases, double adenomas in 5%, and multigland hyperplasia in 15%.
- •
Ultrasound and sestamibi scans are first-line imaging modalities with sensitivities of 80% and 84%, respectively.
- •
4DCT is particularly valuable in cases of nondiagnostic or discordant ultrasound and sestamibi, or after failed neck operation.
Introduction
Primary hyperparathyroidism (PHPT) is an endocrine disorder defined by an elevated or inappropriately normal parathyroid hormone (PTH) level. Secondary hyperparathyroidism is elevated PTH in response to a metabolic alteration in patients with chronic kidney disease, typically due to high blood phosphorus levels, low levels of active vitamin D, or low blood calcium levels. With chronic elevation in PTH levels, true autonomy of the parathyroid glands occurs, leading to tertiary hyperparathyroidism. The role of imaging in hyperparathyroidism is to guide minimally invasive surgical interventions for PHPT. Medical therapy remains the mainstay of management in secondary and tertiary hyperparathyroidism; therefore, the focus of this review is related to PHPT.
The prevalence of PHPT is 1 to 7 cases per 1000 individuals, with higher incidence among female and Black individuals, and the older population. The highest prevalence of PHPT is among postmenopausal women and is estimated to be as high as 3%. Sporadic PHPT is associated with exposure to ionizing radiation in childhood and with chronic lithium use. The clinical presentation is usually nonspecific, and classically summarized as “stones, bones, groans, and moans” in reference to kidney stones, bone pain, abdominal groans from gastrointestinal symptoms, and psychiatric moans from central nervous system effects. Although this remains the clinical presentation in developing countries, the most common presentation in the United States and Europe is incidental asymptomatic hypercalcemia on routine laboratory analysis. Normocalcemic hyperparathyroidism is a variant of PHPT recognized in 2009, usually diagnosed during investigations for low bone mineral density. PHPT is caused by a single enlarged adenoma in 80% of cases, double adenomas in 5% to 10% of cases, multigland hyperplasia in 10% to 15% of cases (usually in familial endocrinopathies), and parathyroid carcinoma in less than 1% of cases.
The parathyroid glands develop from the pharyngeal pouches in the fifth and sixth weeks of gestation and migrate caudally. The superior glands descend only a short distance, and thus their final location is less variable. They are typically located posterior to the thyroid, between the junction of the upper and middle thirds of the thyroid gland, and close to the inferior margin of the cricoid cartilage. The inferior glands migrate for a longer distance, more medially and inferiorly, and are thus more variable in location, and can be found anywhere from the angle of the mandible to the superior pericardium , ( Fig. 1 ). Their classic location is directly posterior to the inferior margin of the thyroid gland (50% of cases) or 1 cm below it (15% of cases). Ectopic locations for either superior or inferior glands include the tracheoesophageal groove, retroesophageal space, and rarely within the thyroid. Additional locations for ectopic superior parathyroid glands include retropharyngeal locations and posterior mediastinum, whereas ectopic inferior glands also can be found within the thymic capsule (38% of ectopic glands), carotid sheath, submandibular region, and down into the mediastinum. , Normal glands are very small in size, measuring 5 × 3 × 1 mm with estimated weight of 15 to 45 mg, and are usually not visualized on imaging. Most people have 4 parathyroid glands, although 2.5% to 15.0% have supernumerary glands that can be located adjacent to the normal parathyroid glands or in the mediastinum and thymic capsule.

Imaging protocols
Imaging modalities used for localizing parathyroid adenomas include ultrasound, 4-dimensional computed tomography (4DCT) and nuclear medicine scintigraphy. Imaging protocols are summarized in Table 1 .
| Protocol parameter | Ultrasound | 4DCT | Scintigraphy |
|---|---|---|---|
| Scan range | Hyoid bone to thoracic inlet | Angle of mandible to carina | Parotid glands to lower heart |
| Patient positioning | Supine with neck hyperextension | Supine | Supine |
| Contrast or radiopharmaceutical | – | 100 mL Omnipaque 350 at 5–6 mL/s | 20 mCi Tc-99m sestamibi |
| Timing | – | Noncontrast Arterial phase (25 s) Venous phase (82 s) | Immediate at 5 min; delayed at 2 h |
| Acquisition | Linear high-frequency transducer | Slice thickness 0.75 mm | Immediate planar Delayed planar and SPECT/CT |
| Reconstruction | – | Axial, coronal, and sagittal 2-mm soft tissue windows | Axial, coronal, and sagittal typically at acquired thickness |
Ultrasound provides the advantages of being relatively inexpensive, using nonionizing radiation, delivering superb image quality in thin patients, having a low carbon footprint, and especially providing concomitant evaluation of the thyroid gland. Its primary disadvantages are poor image quality in obese patients and operator dependency. It is typically performed after placing a pillow under the patient’s shoulders so that there is hyperextension of the neck. A high-frequency linear transducer is used to allow for higher resolution. Additional evaluation of the superior mediastinum can be attempted with a high-frequency curved array transducer, such as the ones used for neonatal head or transvaginal ultrasound. Color Doppler or power Doppler can also help in the diagnosis. The examination initially focuses on the region posterior and inferior to the thyroid gland. Visualization of the paratracheal and paraesophageal regions is improved by turning the patient’s head to the contralateral side and scanning from a lateral approach. When an enlarged parathyroid gland is not detected in the typical locations, survey of potentially ectopic locations also should be performed. ,
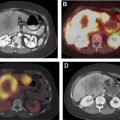
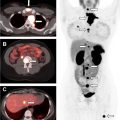
Nuclear scintigraphy for parathyroid adenoma localization is usually performed with a single radiotracer (Tc-99m sestamibi). Immediate and 2-hour delayed planar images are obtained, in addition to an immediate or a delayed single-photon emission computed tomography (SPECT) or SPECT/CT acquisition. Because the most common cause for a false positive diagnosis of parathyroid adenoma on scintigraphy is a thyroid adenoma, a dual tracer subtraction technique can provide a way to characterize the latter. In the dual tracer technique, an additional imaging acquisition is performed with a thyroid imaging agent, such as Tc-99m pertechnetate or I-123, then subtracted from the sestamibi images.
4DCT is another major technique for localizing parathyroid adenomas. It was introduced as a multiphase CT with 4 phases (noncontrast, arterial, venous, and delayed), thus referred to as 4D, the fourth dimension being time. The fourth delayed phase is no longer considered necessary because the 3-phase protocol provides a good balance between radiation exposure and diagnostic performance.
Imaging findings/pathology
Diagnostic Criteria
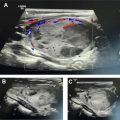
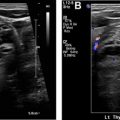
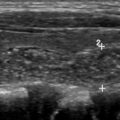
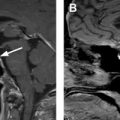
The diagnostic criteria for parathyroid adenomas are listed in Table 2 . On ultrasound, the typical appearance for an enlarged parathyroid is a solid, well-circumscribed homogeneously hypoechoic ovoid structure, with its long axis oriented craniocaudally ( Figs. 2 and 3 ). Less commonly, parathyroid adenomas can be isoechoic to the thyroid, contain focal hyperechoic areas, demonstrate internal heterogeneity, or contain cystic spaces ( Fig. 4 ). Adenomas usually have detectable internal vascularity that may be less than, equal to, or greater than thyroid vascularity. However, when adenomas are small and deep, there may be no detectable flow. In some cases, there is a dominant peripheral vessel inserting or exiting at its pole. A large size of more than 3 cm, lobulated or irregular margins, extensive heterogeneity, or calcifications should raise concern for parathyroid carcinoma, particularly when the patient has more severe symptoms and laboratory abnormalities. The sensitivity of ultrasound for detecting parathyroid adenomas has been reported to range between 65% and 97%, with one meta-analysis concluding an overall sensitivity of 80%. In another meta-analysis, ultrasound had a pooled sensitivity of 76.1% and positive predictive value (PPV) of 93.2% for detecting parathyroid adenomas. Sensitivity for detection of small parathyroid adenomas is particularly good in patients with thin necks ( Fig. 5 ). Sensitivity, however, decreases in obese patients, with multigland disease, after prior neck surgeries, and in the presence of thyromegaly. , Due to lack of an acoustic window, ultrasound also has limited sensitivity in detecting ectopic mediastinal parathyroid glands.
| Ultrasound | Solid well-circumscribed homogeneously hypoechoic ovoid structure |
| Craniocaudal orientation of the long axis | |
| Variable internal vascularity | |
| Scintigraphy | Persistent radiopharmaceutical retention on delayed images |
| Extrathyroidal radiotracer uptake on SPECT images | |
| Tracer-avid soft tissue nodule on fused SPECT/CT images | |
| 4DCT | Early arterial enhancement |
| Early washout on venous-phase images |