PAROTID NONINFECTIOUS INFLAMMATORY CONDITIONS
KEY POINTS
- Imaging, especially computed tomography, sometimes provides critical information in the management of parotid inflammatory pathology by distinguishing infectious and noninfectious etiologies.
- Computed tomography and magnetic resonance imaging may identify changes suggestive of autoimmune sialoadenitis before that diagnosis is established clinically.
- Imaging may reveal important complications of chronic disease, such as lymphoma complicating chronic autoimmune sialoadenitis.
The vast majority of parotid inflammatory conditions do not come to imaging. Imaging may be used to help in medical decision making when the source of disease and/or the question of an infectious or noninfectious parotid region inflammation is raised. Sometimes, low-grade, chronic inflammatory conditions mimic a neoplasm by presenting predominantly as a parotid mass. Imaging in patients with chronic parotid-region complaints may be the first indication of the parotid disease being related to a systemic condition, most often a manifestation of autoimmune disease and less commonly other systemic inflammatory disease as common as sarcoidosis and as rare as Kimura disease. The distinction between infectious disease (Chapter 177) and noninfectious inflammatory disease and neoplasm (Chapter 179) sometimes is blurred, as with the parotid manifestations associated with human immunodeficiency virus (HIV) infection and rheumatoid arthritis.
APPLIED ANATOMY
The important anatomic considerations in parotid (and other major salivary gland) noninfectious inflammatory conditions are mainly confined to the gland and take into account the variations in the gland’s normal relative fat and glandular tissue and the ductal system anatomy. The parotid capsule attachments may result in minor secondary changes in adjacent structures, primarily the external ear and masseter muscle. This anatomy should be reviewed in detail at this point, if necessary, in Chapter 175 and with regard to the facial nerve in Chapter 179. The facial nerve is typically not of such primary interest, as it may be in parotid infectious and particularly in parotid masses. The following anatomic relationships must be understood to ensure that imaging data is incorporated into the assessment and ultimately the treatment of parotid inflammatory conditions:
- Ductal system within the gland and then through the buccal space to its point of drainage in the upper gingivobuccal sulcus across from the second molar
- Attachments of the parotid capsule to the external auditory canal and its commonality with masseteric fascia
- Facial nerve and the anatomic landmarks that relate to the surgical approach to facial nerve preservation
- Parotid lymph nodes
IMAGING APPROACH
Techniques and Relevant Aspects
Computed Tomography
Specific computed tomography (CT) protocols for various indications appear in Appendix A and are discussed in more detail in Chapter 175. Theoretically, calcification might be masked by contrast use, but this does not justify the cost or radiation burden of routine pre- and postcontrast studies. Stones will be clearly identifiable as separate from enhancing structures in the gland in virtually all cases.
Magnetic Resonance Imaging
Specific magnetic resonance (MR) protocols for various indications appear in Appendix B. A steady state image may be included that allows for MR depiction of the ductal system, sometimes referred to as MR sialography.
Radionuclide Studies
Radionuclide studies are not used routinely for the evaluation of parotid gland inflammatory conditions.
Ultrasound
Standard high-resolution scanning techniques are used as described in Chapter 4. Ultrasound (US) and conventional sialography can be used sometimes as an adjunct to sialoendoscopy for evaluating the ductal complications of some of these conditions.
Conventional Sialography
Conventional sialography has been used very selectively since the 1980s and most recently in conjunction with endoscopic treatment of main duct pathology.
Pros and Cons
General Approach
Magnetic Resonance and Computed Tomography
MR and CT are the most-used imaging studies to evaluate an acute or subacute parotid region inflammation, since injection of the ductal system even with nonionic and low osmolarity contrast can worsen the condition. CT is preferred for its simplicity and to avoid the insensitivity of MRI to stones both within the ductal system and the parenchyma. The dimension of ductal system visualization added by MR sialography is typically not of enough critical value in cases of acute inflammation to supplant CT.
In chronic inflammatory conditions, MR might be a first choice. Both CT and magnetic resonance imaging (MRI) might be deferred in favor of US and sialoendoscopy in the hands of experienced groups when main duct pathology is believed to be the most likely contributing factor. MR sialography is a useful adjunct to depict the ductal system but in general is not justified as the sole indication for this very expensive study.
CT is by far the most sensitive test for detecting calcified parotid stones both in the ductal system and the gland parenchyma (Chapter 177). US is adequate for many cases of when only main duct pathology is the diagnostic and treatment focus.
Ultrasound and Conventional Positive Contrast Sialography
US may be used to determine whether there is ductal dilatation or stones. The US parenchymal changes seen in these diseases are nonspecific. Moreover, all of the major salivary glands are not studied. Other regional anatomy that may be of interest, such as the cervical lymph nodes and lacrimal glands, are either not studied or are more laborious to include in a comprehensive head and neck imaging examination than with CT and MRI.
US alone may be enough imaging for triage to appropriate care in selected cases in the hands of experienced sonographers. This is especially true where teams that triage with US for potential follow-up sialoendoscopy are in place. Sialography may also fit well into such a triage scheme for main duct disease, possibly following a nondefinitive US in the diagnostic hierarchy.
Radionuclide Studies
Radionuclide studies are not used routinely for the evaluation of parotid gland inflammatory conditions.
Specific Indications
Specific indications for parotid gland inflammatory conditions include the following:
- Define the origin and extent of a parotid region inflammation, determining whether the process arises within the parotid gland or from an extrinsic source.
- Define whether the processes are likely of noninfectious or infectious cause.
- Identify ductal changes or sialolithiasis that might contribute to a diagnosis and treatment plans.
- Establish whether the changes may be part of a generalized major salivary gland condition and/or possibly related to a systemic inflammatory process.
Controversies
US may be promoted as a starting point in the evaluation of parotid inflammatory disease; however, the sonographic evaluation of the deep lobe is fundamentally limited by the bony confines of the mandible and mastoid. Using US as a starting point is a very good approach but only in experienced hands. Outside of that context, US becomes potentially cost additive and nondefinitive.
MR sialography had been promoted as a screening tool for Sjögren disease in the past. However, there are more cost-efficient and reliable means of establishing such a diagnosis. Sjögren disease is primarily a clinical diagnosis that is confirmed by serologic studies. In serologically negative patients with highly suspicious findings of dry eyes and mouth, imaging may aid in medical decision making. Lip biopsy is simple, not expensive, and a generally accepted means of diagnosis. Unless there is a risk of significant reaction to iodinated contrast, MRI should defer to CT if an advanced imaging technique is necessary for medical decision making.
The value of using a combination of sialography and endoscopic treatment of ductal pathology is becoming more widespread. This is not controversial but does require a careful coordinated effort between the sialendocopist and the imaging team.
SYSTEMIC AND FOCAL NONINFECTIOUS INFLAMMATORY CONDITIONS, RADIATION-INDUCED SIALOADENITIS, AND TRAUMATIC SIALOADENITIS
Etiology
Systemic diseases that might present in and/or involve the parotid glands are mainly those due to autoimmune disease (Figs. 178.1–178.6), essentially Sjögren syndrome, Sjögren disease, and sarcoidosis (Fig. 178.7). Other more rare etiologies include Wegener granulomatosis (WG) and Rosai-Dorfman disease. Kimura disease may present as focal parotid disease (Fig. 178.8).
Radiation-induced sialoadenitis is a regional disease but is considered here because it typically involves all of the major salivary glands with a natural history that may be the same as autoimmune disease, although on a comparatively accelerated timetable (Fig. 178.9).
Posttraumatic sialoadenitis can be caused by blunt or penetrating injury (Fig. 178.10) and is sometimes iatrogenic (Fig. 178.11).
Prevalence and Epidemiology
These factors are described for autoimmune disease in Chapter 20, sarcoidosis in Chapter 18, WG in Chapter 17, and the presumed reactive and possibly inflammatory lymphoproliferative disorder Kimura disease in Chapter 26.
Clinical Presentation
Parotid inflammatory conditions will present with unilateral (often asymmetric) or bilateral pain, tenderness, and enlargement of the parotid. Symptoms may be exacerbated during meals. There may be associated parotid and cervical reactive lymphadenopathy. In few cases, these symptoms may be less apparent and the predominant complaint may be a parotid-region mass, parotid-region discomfort, or otalgia associated with meals. In trauma and radiation-induced sialoadenitis, the cause will be known. In trauma, saliva leakage into the soft tissues can cause a great deal of inflammation mimicking abscess.
Pathophysiology and Patterns of Disease
The morphology of infectious and inflammatory diseases is discussed in detail in Chapters 13 through 16. Those inflammations unique to the parotid and submandibular glands include ductal obstructive patterns that may manifest along the entire main parotid duct or more focally when strictures and/or stones are present. The wall of a dilated ductal system may enhance (Figs. 178.1–178.6). The more proximal ductal system draining the gland parenchyma may also be dilated. Terminal acinar destruction may produce parenchymal cysts that communicate with the main ductal system (Figs. 178.1 and 178.2).
Glandular parenchymal changes are variable but constitute a major component of the imaging findings. Autoimmune disease is, to some extent, a model for these chronic parenchymal conditions. The basic disease process is due to immune activation and extensive lymphocytic infiltration that causes acinar cell and ductal epithelium destruction. In HIV disease, it is the T lymphocytes that aggregate this disease as opposed to polyclonal activated lymphocytes. The other systemic diseases generally follow a similar final common pathway of revision of glandular architecture. The ducts become blocked by inspissated debris and produce acinar pseudocysts. Cysts eventually expand as the parenchyma is destroyed, and broad zones of lymphocyte accumulation result in the solid masslike components that are difficult to tell from lymphoma. Diffuse enhancement may be seen in the more active phase of the disease (Figs. 178.1 and 178.2). This is most consistently observed in acute and subacute radiation-induced sialoadenitis (Fig. 178.9) but is common to all chronic inflammatory diseases to some extent and/or degree.
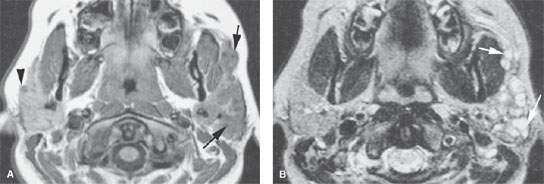
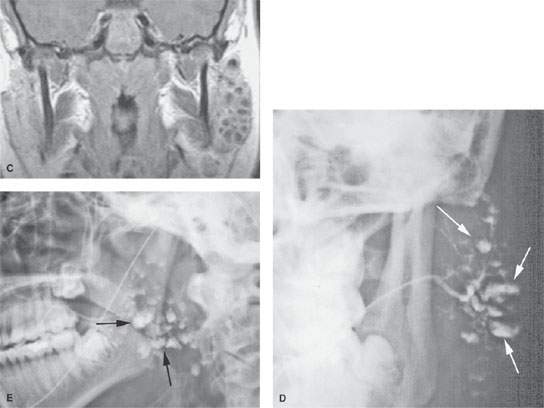
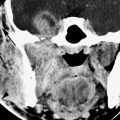
FIGURE 178.1. Magnetic resonance (MR) and conventional sialography studies in a patient with autoimmune sialoadenitis presenting predominately on the left side. A: Contrast-enhanced T1-weighted (T1W) image showing minimal disease on the right side that was asymptomatic and characterized mainly by diffuse enhancement of the gland and one of only few parenchymal cysts (arrowhead). On the left side, the gland is enlarged and contains multiple cystic areas scattered through the gland parenchyma, involving the accessory lobe as well as the main portion of the parotid gland (arrows). B: T2-weighted image to correlate with (A) showing the largely cystic replacement of the gland parenchyma (arrows). C: T1W coronal image somewhat delayed from the axial image seen in (A) showing the very extensive parenchymal cysts on the more involved left side. D, E: A sialogram done on this same patient showing that there is good filling of the normal parotid duct, which appears normal. The branch parotid ducts all show narrowing and irregularity consistent with the chronic inflammatory disease. Large cystic spaces (arrows) are in communication with the ductal system due to terminal acinar destruction. These changes explain at least a portion of the cystic changes seen on the MR study. In (E), a delayed image with the cannula removed shows stagnant contrast in the parenchymal cysts (arrows).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree