Part 4 Mesentery and Vascular
Case 123

Clinical History
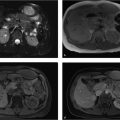
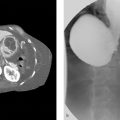
A 52-year-old female with general abdominal pain, 1 week after surgery (▶Fig. 123.1).
Key Finding
Curvilinear high density.
Top 3 Differential Diagnoses
Textiloma: Textiloma is also known as gossypiboma and refers to a cotton-based mass left in the body after a surgical procedure. The true incidence is not known, but estimated to be 1 in 1,000 to 1,500 abdominal surgeries. Textilomas account for 50% of malpractice claims for retained foreign bodies. On X-ray, the radiopaque marker strip of the surgical sponge is easily seen as a high density within the mass, whereas the remaining cotton sponge is not well seen. This radiopaque marker strip appears “ribbon-like” in shape. Complications result from the exudative and aseptic fibrotic body response leading to abscess, fistula formation, bowel obstruction, malabsorption, and gastrointestinal hemorrhage. Delays in diagnosis may increase mortality and morbidity. Surgical removal is the definitive treatment.
Ingested pills/foreign bodies: The key to diagnosis is noting the irregular and nonanatomical morphologies. Swallowed pills/objects will most likely move location on repeat studies. Metallic objects will be more radiopaque compared to bones and calcific densities.
Atherosclerosis: Atherosclerosis typically appears as curvilinear parallel lines of calcification in the expected location of arteries.
Diagnosis
Textiloma, retained surgical sponge.
✓ Pearls
Retained surgical sponges are identified by noting their radiopaque marker strip.
Gossypiboma can be seen in any part of the body.
Calcified vessels are not as dense as metal or high density foreign bodies.
Suggested Readings
Guelfguat M, Kaplinskiy V, Reddy SH, DiPoce J. Clinical guidelines for imaging and reporting ingested foreign bodies. AJR Am J Roentgenol. 2014; 203(1):37–53 Manzella A, Filho PB, Albuquerque E, Farias F, Kaercher J. Imaging of gossypibomas: pictorial review. AJR Am J Roentgenol. 2009; 193(6, Suppl):S94–S101Case 124

Key Finding
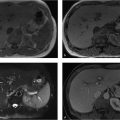
Portal vein enlargement.
Top 3 Differential Diagnoses
Portal hypertension: Elevated portal pressure is due to increased resistance to portal blood flow, most commonly from liver cirrhosis. Defined as absolute portal venous pressure of more than 10 mm Hg or a gradient between portal and systemic veins of greater than 5 mm Hg. Portal hypertension is classified into three categories: presinusoidal from a portal vein or splenic vein thrombus, sinusoidal from liver disease (cirrhosis being the most common, hepatocellular disorder, or hepatic tumor), and postsinusoidal from right heart failure or constrictive pericarditis. Cross-sectional imaging features include portal vein dilation of more than 13 mm (a direct finding), ascites, splenomegaly, varices, recanalization of paraumbilical vein, mesenteric edema, and gallbladder wall thickening. Ultrasound findings include slow (< 15 cm/s) or reversed (hepatofugal) blood flow in the portal vein. There is a high risk of variceal bleeding with portosystemic gradient of more than 12 mm Hg.
Cavernous transformation of portal vein: This process occurs as the sequelae of chronic/persistent portal vein thrombus. It is the replacement of a normal single portal channel into the porta hepatis with numerous tortuous venous channels from dilated collateral veins (thought to be paracholedochal veins). The transformation process takes a variable amount of time, from 1 week to a year. Ultimately, cavernous transformation results in presinusoidal portal hypertension.
Superior vena cava (SVC) obstruction: There are many etiologies of SVC obstruction with the most common being tumor invasion (lymphoma) and thrombosis (commonly related to indwelling lines and tubes). SVC obstruction can result in “downhill” varices from collateralization to redirect the return of blood to the inferior vena cava (IVC) and liver.
Diagnosis
Portal hypertension, sinusoidal due to cirrhosis.
✓ Pearls
Transjugular intrahepatic portosystemic shunt (TIPS) is used to reduce portal pressure in patient with intractable ascites or variceal bleeding.
Remember hepatopetal flow is toward liver, hepatofugal flow is away from the liver (think of a fugitive running away from the liver).
The most common cause of portal hypertension is cirrhosis, and the most common cause of cirrhosis is EtOH abuse. The most serious complication is esophageal or gastric variceal bleeding.
Suggested Readings
Gerstenmaier JF, Gibson RN. Ultrasound in chronic liver disease. Insights Imaging. 2014; 5(4):441–455 Lee JY, Kim TY, Jeong WK, et al. Clinically severe portal hypertension: role of multi-detector row CT features in diagnosis. Dig Dis Sci. 2014; 59(9):2333–2343 Liu CH, Hsu SJ, Liang CC, et al. Esophageal varices: noninvasive diagnosis with duplex Doppler US in patients with compensated cirrhosis. Radiology. 2008; 248(1):132–139Case 125

Key Finding
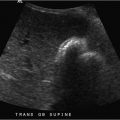
Right lower quadrant lymph nodes.
Top 3 Differential Diagnoses
Mesenteric adenitis: Typical presentation is a cluster of three or more lymph nodes (5 mm or larger short axis) in the right lower quadrant. The nodes are homogenous in attenuation and classically seen anterior to the right psoas but may be in the root of the mesentery. Primary mesenteric adenitis is defined as having no identifiable inflammatory cause. Secondary mesenteric adenitis is characterized by the concomitant intra-abdominal inflammatory process.
Lymphoma: Lymphoma may result in lymphadenopathy anywhere in the body. Early in the disease course, the nodes may be small, scattered, and discrete. As the disease progresses, the lymph nodes coalesce into soft-tissue masses. The attenuation of the nodes tends to match soft tissue and enhance homogenously.
Inflammatory bowel disease: Mesenteric adenopathy is frequently seen with both Crohn’s disease and ulcerative colitis. Crohn’s disease is more common. Inflammatory changes of the small or large bowel are usually present but not necessary. Nodes can be seen in the mesenteric root, the mesentery periphery, and the right lower quadrant. The nodes are described as enlarged but not massive. On CT, the nodes are typically soft-tissue attenuation and enhance homogenously.
Additional Diagnostic Considerations
Inflammation: Common causes of inflammatory mesenteric lymphadenopathy are appendicitis, diverticulitis, and pancreatitis. In appendicitis, the presence of enlarged right lower quadrant nodes with an abnormal appendix is diagnostic. Appendiceal carcinoma is rare but can mimic appendicitis, especially if perforated (associated adenopathy is larger). Lymphadenopathy related to diverticulitis is usually localized to the area of inflamed colon. Nodes tend to be reactive and small. Perforated colon carcinoma can also mimic diverticulitis and the presence of enlarged nodes may be a clue. Finally, pancreatitis usually results in retroperitoneal and peripancreatic adenopathy. However, as the inflammatory changes of pancreatitis can be extensive any location of abnormal lymph nodes in the abdomen or pelvis is possible.
Diagnosis
Mesenteric adenitis.
✓ Pearls
Lymph node number, size, and distribution are all important indicators of etiology.
Mesenteric adenopathy in a patient with lymphoma does not always indicate active disease.
Incidence of primary mesenteric adenitis is considered low.
Suggested Readings
Johnson CD, Schmit G. Mayo Clinic Gastrointestinal Imaging Review. Rochester, MN: Mayo Clinic Scientific Press; 2005 Lucey BC, Stuhlfaut JW, Soto JA. Mesenteric lymph nodes seen at imaging: causes and significance. Radiographics. 2005; 25(2):351–365 Macari M, Hines J, Balthazar E, Megibow A. Mesenteric adenitis: CT diagnosis of primary versus secondary causes, incidence, and clinical significance in pediatric and adult patients. AJR Am J Roentgenol. 2002; 178(4):853–858Case 126

Key Finding
Peritoneal linear enhancement.
Top 3 Differential Diagnoses
Peritonitis: Any inflammatory process within the abdomen can cause abnormal peritoneal enhancement including perforated abdominal viscus, appendicitis, or inflammatory bowel disease. Other infections/inflammatory conditions to consider include spontaneous bacterial peritonitis and pelvic inflammatory disease. Abnormal peritoneal enhancement from an adjacent inflammatory process or infection typically causes smooth enhancement without nodularity.
Peritoneal carcinomatosis: Irregular enhancement of the peritoneum with nodularity is the typical presentation of peritoneal carcinomatosis. Peritoneal carcinomatosis is classically considered with gastrointestinal and ovarian malignancies but can be seen with many other types of tumors including the appendix, breast, or gallbladder. Ascites typically occurs in the setting of peritoneal carcinomatosis and should prompt close evaluation of the peritoneum, especially if it is a new finding in a patient with a known malignancy.
Pseudomyxoma peritonei: Also known as “jelly belly,” pseudomyxoma peritonei occurs when the peritoneal cavity is coated with thickened gelatinous material and is classically associated with mucinous carcinomas secreting mucous into the peritoneal cavity. The most common mucinous neoplasm that causes pseudomyxoma peritonei is mucinous adenocarcinoma of the appendix. Radiographic findings overlap large abdominal ascites, although pseudomyxoma peritonei causes more displacement of peritoneal organs and may demonstrate curvilinear calcifications.
Additional Diagnostic Considerations
Sclerosing encapsulating peritonitis: It is an inflammatory condition seen in patients undergoing peritoneal dialysis, but it can also occur with idiopathic fibrosis of the peritoneum. Imaging findings typically manifest as thin abnormal enhancing peritoneum with loculated fluid collections.
Diagnosis
Peritonitis secondary spontaneous bacterial peritonitis.
✓ Pearls
Any abdominal inflammatory or infectious process may cause abnormal peritoneal enhancement.
Malignant or metastatic involvement demonstrates nodular enhancement of the peritoneum.
Pseudomyxoma peritonei is classically associated with mucinous adenocarcinoma of the appendix.
New onset abdominal ascites, especially in a patient with a known malignancy should prompt detailed evaluation of the peritoneum to evaluate for peritoneal carcinomatosis.
Suggested Readings
Federle MP, Jeffrey RB, Woodward PJ, Borhani A. Diagnostic Imaging: Abdomen. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009 Filippone A, Cianci R, Delli Pizzi A, et al. CT findings in acute peritonitis: a pattern-based approach. Diagn Interv Radiol. 2015; 21(6):435–440 Levy AD, Shaw JC, Sobin LH. Secondary tumors and tumor-like lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009; 29(2):347–373Case 127

Key Finding
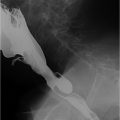
Abdominal aortic cleft.
Top 3 Differential Diagnoses
Aortic dissection: Aortic dissection occurs when blood enters the media layer of the aortic wall though an intimal defect. CT is the best imaging modality and demonstrates an intimal flap resulting in a true and false lumen. The true lumen is contiguous with the normal portion of aorta while the false lumen may be thrombosed or demonstrate delayed flow. Radiographs may demonstrate displaced intimal calcifications. Type A dissection always involves the thoracic ascending aorta only and is treated surgically. If left untreated, there is a 50% mortality rate within 48 hours. A type B dissection involves the descending thoracic aorta and/or arch and is treated medically. It is important to delineate the relationship between the true lumen and the origins of the abdominal aortic branches.
Intramural hematoma: This is caused by arterial bleeding from the vasa vasorum into the media layer resulting in hemorrhage deposition within the wall. There is no appreciable intimal flap or false lumen.
Penetrating aortic ulcer (PAU): PAU occurs when an atheromatous plaque ulcerates and disrupts the internal elastic lamina and extends into the aortic media. On CT, it appears as a contrast filled protrusion into an intramural hematoma, which is isoattenuated to the aorta. There is no appreciable intimal flap or false lumen. This is most commonly seen in the thoracic aorta.
Additional Diagnostic Considerations
Pseudodissection: The aortic pulsation can create artifact that mimics an intimal flap and typically involves the left anterior and the right posterior aspects of the ascending aorta.
Diagnosis
Aortic dissection.
✓ Pearls
Aortic dissection demonstrates an intimal flap with a true and false lumen.
Type A dissection is surgical due to involvement of the aortic root and possibly the coronaries.
Marfan’s syndrome, Ehlers–Danlos syndrome, and other connective tissue diseases are often associated with aortic dissection.
PAU is focal and isoattenuated to the aorta.
Suggested Readings
Bailkousis NG, Apostolakis EE. Penetrating atherosclerotic ulcer of the thoracic aorta: diagnosis and treatment. Hellenic J Cardiol. 2010; 51(2):153–157 Macura KJ, Corl FM, Fishman EK, Bluemke DA. Pathogenesis in acute aortic syndromes: aortic dissection, intramural hematoma, and penetrating atherosclerotic aortic ulcer. AJR Am J Roentgenol. 2003; 181(2):309–316 Sebastià C, Pallisa E, Quiroga S, Alvarez-Castells A, Dominguez R, Evangelista A. Aortic dissection: diagnosis and follow-up with helical CT. Radiographics. 1999; 19(1):45–60, quiz 149–150Case 128

Key Finding
Focal mesenteric fat stranding.
Top 3 Differential Diagnoses
Omental infarct: Typical presentation is a fatty mass with inflammatory fat-stranding within the right colon. Larger areas of fatty mass may demonstrate gas or fat fluid levels. The adjacent colon should be normal. This is a benign self-limited condition that is treated conservatively with analgesics only. The presentation can be clinically confusing as patients usually present with right lower quadrant pain and fever. Therefore, exclusion of surgically treated etiologies (appendicitis, cholecystitis) or etiologies requiring antibiotics (uncomplicated diverticulitis) is needed. The abnormal CT findings usually resolve in 1 to 2 months.
Diverticulitis: Mesenteric fat stranding is present in a pericolonic distribution. Colonic diverticula are present with associated segmental bowel wall thickening. The sigmoid colon is the most common location. Trace extraluminal fluid and small foci of gas may be seen. Acute complications include abscess formation and perforation. Fistulas may result as a chronic complication.
Epiploic appendagitis: Epiploic appendages are protrusions of fat from the surface of the colon. Torsion of these appendages with secondary ischemia leads to the condition. Classic imaging findings are focal fat stranding with peripheral rim centered away from the colon and absence of or minimal bowel wall thickening. The sigmoid colon is the most commonly affected segment of colon and the inflammation is usually seen anteriorly. A central hyperattenuating dot may be present representing the thrombosed vascular pedicle.
Additional Diagnostic Considerations
Mesenteric panniculitis: It is of an unknown etiology with hazy fat stranding usually at the root of the mesentery. This location is the most useful characteristic when differentiating from other etiologies. Lymph nodes may be present. Other imaging features of mesenteric panniculitis include tumoral pseudocapsule and fat halo sign.
Diagnosis
Omental infarct.
✓ Pearls
An omental infarct resulting from prior surgery or trauma may be atypical in location.
Colon cancer may mimic diverticulitis and follow-up CT may be needed to document resolution.
A fatty lesion with inflammatory changes in the right lower quadrant is considered to be omental infarct.
Suggested Readings
Johnson CD, Schmit G. Mayo Clinic Gastrointestinal Imaging Review. Rochester, MN: Mayo Clinic Scientific Press; 2005 Kamaya A, Federle MP, Desser TS. Imaging manifestations of abdominal fat necrosis and its mimics. Radiographics. 2011; 31(7):2021–2034 Singh AK, Gervais DA, Hahn PF, Sagar P, Mueller PR, Novelline RA. Acute epiploic appendagitis and its mimics. Radiographics. 2005; 25(6):1521–1534Case 129

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree