(1)
Clinical Department, Urals Research Centre for Radiation Medicine, Chelyabinsk, Russia
Abstract
Pathological–anatomical characteristics of CRS have not yet been adequately studied. Fragmentary information available to date includes data on morphological changes in different organs which have mostly resulted from the follow-up of the personnel of atomic plants and animal studies. The data obtained are mainly indicative of dystrophic changes in the internal organs and in the CNS in the period of CRS development in persons with severe form of the syndrome.
Pathological–anatomical characteristics of CRS have not yet been adequately studied. Fragmentary information available to date includes data on morphological changes in different organs which have mostly resulted from the follow-up of the personnel of atomic plants and animal studies. The data obtained are mainly indicative of dystrophic changes in the internal organs and in the CNS in the period of CRS development in persons with severe form of the syndrome.
4.1 Circulatory System
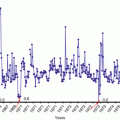
As it has already been observed, the development of CRS is extremely dynamic, and it depends on the exposure dose rate to critical organs. Under the conditions of a continuous exposure at doses exceeding the threshold values for individual organs, it is functional insufficiency of all hematopoietic organs (BM, spleen, etc.) that is progressing first of all. The manifest changes in hematopoiesis (Chap. 6) are mostly accounted for structural changes in the BM.
Studies of BM during the early stages of CRS development helped identify cases with a considerable decrease in the majority of myelokaryocytes (as a rule, <70 · 109/l), particularly of megakaryocytes and megakaryoblasts. Structural changes in megakaryocytes were observed in the absence of manifest azurophilic granularity, cytoplasmic basophilia, and impaired formation of thrombocytes. In a number of cases, disintegration of thrombocytes and the presence of large numbers of megakaryocyte nuclei were noted in BM aspirates. The leukoerythroblastic index and neutrophil maturation index were usually reduced to <3 and <0.6, respectively. Cytological changes in neutrophils resembling pycnosis and cytoplasm vacuolization were observed in all cases of CRS with severe clinical course and in a proportion of CRS cases of moderate severity. No essential changes in the erythroid cell lineage were observed during this period. In individual cases megaloblasts occurred which, according to the authors, testified to the onset of BM regeneration process. The course of regeneration may involve formation of pathological forms (megaloblasts, plasmocytes) and derangement of the maturation process. More than half of the persons, mostly those with severe CRS, exhibited, as a rule, an increase in the number of reticular cells (>1 %) and plasmocytes (>2 %). Over the ensuing months (up to month 12 of the follow-up after the termination of occupational exposure to IR), half of the patients showed increased counts of myelokaryocytes, while no dynamic changes were noted in the remainder patients. On the whole, the number of megakaryocytes increased in 70 % of patients. Cytological changes noted in neutrophils of persons with CRS decreased, no megaloblasts were registered, and leukoerythroblastic ratio usually remained decreased. Distinct regenerative changes (Baysogolov 1961) were obvious.
In the terminal stage of CRS, almost complete lack of hematopoietic cells was observed in the BM. Diffuse and focal proliferation of reticular cells and hemocytoblasts1 was observed, in some areas plasmocytes predominated, and occasionally gigantic cells and mitoses were noted. Atrophy of lymphoid tissue, spleen, lymph nodes, and tonsils was marked too. The proliferation of non-differentiated reticular cells and hemocytoblasts was predominantly seen in the spleen (Lemberg 1959).
Of considerable interest in terms of morphological changes in the organs in CRS cases were the autopsy findings described by GD Baysogolov and VK Lemberg in 1959. The first signs of CRS including leukopenia and thrombocytopenia in persons with CRS became evident as early as the first year of the occupational exposure to IR due to which a person was transferred to a job which did not involve radiation exposures. In spite of that, the patient’s hematological symptoms were progressing. The administered therapy (Campolon,2 sodium nucleinate, hemotransfusions) provided some improvement of the patient’s condition and his objective status 18 months later after the termination of exposure to IR. Over this period, the patient’s peripheral blood count improved and the signs of asthenia subsided. His leukocyte count increased up to 3.0–4.0 · 109/l and the thrombocyte count reached 100.0–150.0 · 109/l. However, relative and absolute neutropenia was still manifest, and absolute eosinophilia persisted (10–11 %) too. No substantial changes were manifested by the erythroid cell lineage.
The period of relative well-being lasted for about 9 months, but later on the patient’s condition started to gradually deteriorate again. The course of the disease was aggravated by infectious complications of inflammatory character: aphthous stomatitis, carbuncle of the suprapubic area, and frequent exacerbations of tonsillitis. During that period, the peripheral blood was characterized by reduction of leukocyte counts to 1.8–2.5 · 109/l, relative and absolute neutropenia became more pronounced, and relative lymphocytosis developed. Blood thrombocyte counts decreased to 30.0–60.0 · 109/l. A gradual decline in the content of hemoglobin (to 92 g/l) and erythrocytes (to 3.0 · 1012/l) was observed. Persistent moderate reticulocytosis was evident. The symptoms of organic damage to the nervous system manifested by general increase in the response of tendon and periosteal reflexes persisted, but there were no signs of progression.
In spite of the repeated long-term courses of treatment using a complex of antibiotics, whole blood and packed leukocyte transfusions, vitamin B12, folic acid, Campolon, sodium nucleinate, and other preparations administered to the patient in clinical settings, his condition continued to deteriorate. In the fourth year of his illness, after one of the successive exacerbations of tonsillitis accompanied by fever, the patient’s condition took a dramatic turn to the worse. General weakness and paleness of skin were observed. During the same period, a sharp decrease in the content of hemoglobin and erythrocytes (0.9 · 1012/l) in the peripheral blood was noted in the absence of any clinical signs of hemorrhage. Over the subsequent 10 months (until the patient’s death), a profound inhibition of hematopoiesis accompanied by symptoms of sepsis (chills, remittent fever, purulent foci in the skin of the face and body) was observed. The symptoms also included petechial hemorrhages in the oral mucous membranes, friability of the gums, hemorrhagic diatheses, and epistaxes were noted, while somewhat later, 2.5–3 months before death, the patient developed signs of impaired cardiac function (edema, breathlessness, tachycardia). Further on, the patient developed a severe form of colitis (diarrhea and intestinal pains) and pronounced hypoproteinemia. Cytopenia continued to build up. The patient died of aggravating heart failure and pulmonary edema at the end of the fourth year after the termination of exposure.
This case of death from progressive cardiac failure and pulmonary edema in a person with CRS at the formation stage provided an opportunity to identify pronounced manifestations of hemorrhagic diathesis. BM in flat bones was scanty, semiliquid, and of pink-yellowish color. The fatty BM of the tubular bone diaphyses was replaced by moist pink-grayish tissue. The spleen was enlarged (500 g) and compact, with traces of several ischemic infarctions.
As for the remainder internal organs, the findings included signs of pronounced dystrophic changes. In addition, small subarachnoid hemorrhages and pronounced brain edema were observed (Baysogolov and Lemberg 1959).
Microscopic examinations of the BM revealed paucity of cellular elements which were extensively represented by focal and diffuse aggregations of reticulocytes, plasmocytes, and hemocytoblasts. Mitoses were frequently identified in the BM. In the remaining small foci of myeloid cells, band and segmented cells along with myeloblasts and myelocytes were encountered. Single gigantic cells with nuclei of rounded or irregular shape and homogeneous oxyphilous protoplasms also occurred. Very few cells of the erythroid lineage were noted in the BM.
The examination of the spleen revealed atrophy of lymphatic follicles which mostly consisted of lymphoblasts and reticular cells; the number of mature lymphocytes was rather small. There were numerous cells with observable homogenous lilac-reddish (Pappenheim’s painting technique) cytoplasm and a rounded nucleus. Similar cells, occurring as foci of aggregated cells, or as diffusely scattered cells, can be seen in the red pulp imbibed by erythrocytes. In the same region solitary gigantic polynuclear cells occurred. Pronounced hemosiderosis was registered. Connective tissue trabeculae were edematous and loose. The walls of the central arteries were homogenized and the endothelium was cast off.
The lymphatic nodes were significantly reduced in size due to depletion of mature lymphocytes; however, a lot of plasmocytes were observed. No growth centers were seen. Mitoses were not encountered. A manifest hemosiderosis was noted.
It was revealed in the course of the postmortem examination that the deceased person with CRS had developed well-defined dystrophic changes in hepatic cells to the extent of complete atrophy in the central regions of the lobules, regenerative phenomena in the periphery (formation of cingular bile ducts), and focal infiltrates consisting of lymphoid-reticular cells with an admixture of segmented leukocytes. Also, hemosiderosis of Kupffer cells and hepatic and reticular cells was observed. No sclerotic phenomena were detected. Intertrabecular argyrophilic fibers were well marked.
The examination of the stomach revealed moderate atrophy of the mucous membrane. Small hemorrhages were observed in the mucous membrane of the small intestine. The findings in the large intestine included atrophy of lymphatic follicles and ulceration of the mucous membrane with extensive infiltrates involving plasmatic, reticular, and segmented leukocytes.
The postmortem examination of the brain revealed small perivascular hemorrhages in all parts of the organ. A pronounced pericellular edema was clearly manifested. Substantial dystrophic changes were observed in individual ganglionic cells (chromatolysis, basophilic incrustation, wrinkling). A substantial accumulation of lipofuscin in the ganglion elements was observed.
The pathological findings in the heart included thinning of muscle fibers, edema of the interstitial connective tissue, and small perivascular hemorrhages. The findings in the kidneys consisted of pronounced dystrophic changes in the epithelium of convoluted tubules and atrophy of seminiferous tubules. In the kidneys and lungs, numerous foci of purulent-inflammatory processes were seen. There were large adenoma-like nodes and cells with dystrophic changes in the adrenal glands.
The patient’s premortal condition was characterized by septicopyemia associated with progressive hypoplasia of the normal hematopoietic tissue of the BM. However, in this case, along with depletion of myeloid and particularly erythroid elements, some hyperplasia of immature cells was observed too; it was especially marked in the BM of tubular bone diaphysis. The examination of the patient’s lymphatic tissue showed severe atrophy (Baysogolov and Lemberg 1959).
The examination of the BM trepanobiopsy specimens indicated that the composition of the BM had been recovering with time after the exposure. However, the BM structure had for a long time persisted as substantially impaired, with clearly marked nonuniform distribution of the RBM and fatty tissue, foci of plasmatic impregnation, focal sclerosis, hemorrhages, areas of complete loss of BM cells, and, also, areas of proliferating homogeneous reticular undifferentiated cells (Vorobyov and Shakhmatov 1970).
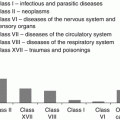
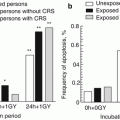
Even 20–25 years later, persons with CRS attributable to external γ-radiation and body intakes of 239Pu exhibited signs of reduced BM cellularity due to parenchymal sclerosis and fatty BM atrophy which represented the key causes of persistent cytopenia at late time after exposure. Up to 70 % of patients showed a reduced number of BM cells (by 60–80 % lower than that shown by the controls) which is regarded as a sign of focal BM hypoplasia. Such patients were noted to develop large zones of cell-free sclerosis, stromal edema and reduction in the active hematopoiesis zone. The type of fatty tissue distribution was also suggestive of the micro-focal nature of BM hypoplasia in CRS. Histological examinations of BM samples revealed micro-focal hypoplasia in 16 % of patients, and 22 % of the examined patients manifested dysplasia of bone tissue (a high level of dysplastic alterations). Absolute reduction in the cellularity of the granulocytic and erythrocytic cell lineages was noted. Thus, incomplete recovery of BM structure served as a basis for cytopenic syndrome development in persons with CRS at late time after exposure. This is demonstrated by reduced BM cellularity and its mosaic structure resulting from fatty atrophy and sclerosis caused by α-radiation, especially in the zones with plutonium accumulation (Vyalova et al. 1980).