References
Year
N patients
Actuarial survival time (years)
PSA-free survival rate (%)
pT1c
pT2
pT2a
pT2b
pT2c
Stamey et al. (4)
1988–1996
366
5
70
72
37
27
Desireddi et al. (5)
1983–1991
3,456
10
70
1992–2003
10
86
Ohori et al. (6)
1983–1999
593
5
94.9 (pT1–2 N0)
10
92.9 (pT1–2 N0)
Amling et al. (10)
1987–1993
2,782
5
82 (pT1c–T2)
10
68
Hernandez et al. (7)
1983–2005
2,551
5
99.7 (pT2 and Gleason Score ≤6)
10
99.1
15
98.7
Bianco et al. (8)
1990–1999
536
96 (pT1–T2)
Chun et al. (9)
1992–2005
2,708
5
93
88 (pT2b–c)
Polascik et al. (11)
1988–1995
5
78
68
1996–2000
 527
52710
82
74
2001–2006
15
94
80
Several studies have identified significant pathologic stage migration favoring early disease, including unilateral pT2a/pT2b cancer [1, 11, 13–16]. There has been disagreement as to whether the AJCC 2002 pathologic stage classification should be amended [17–20]. At present, pathologic T2 disease is subclassified as T2a, T2b, and T2c depending on the volume or laterality of the cancer present. Since pathologic T2b disease is infrequently encountered, it has been brought into question as to whether it would be better to have two subcategories, namely, new T2a and T2b categories representing unilateral and bilateral disease, respectively. Caso et al. recently identified 1990 men who underwent radical prostatectomy between 1988 and 2007 and had pathological T2 a, b, and c disease [13]. The authors concluded that pT2a prostate cancer had significantly higher biochemical disease-free survival in univariate but not in multivariate analysis (Fig. 4.1). This issue of pT2 subdivisions will likely remain a matter of debate.
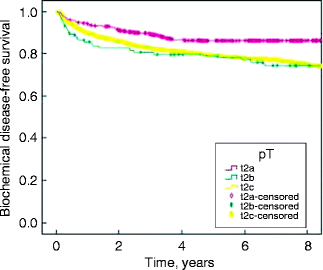

Fig. 4.1
Kaplan–Meier biochemical disease-free survival curves for all pT2 subdivisions (from Caso et al. [13], with permission)
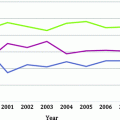
In a study reporting on 3,456 men treated with radical prostatectomy comparing the pre-PSA era (1983–1991) to the PSA era (1992–2003), the proportion of patients with organ-confined disease increased from 64% to 69%, while the frequency of men with advanced-stage disease (seminal vesicle invasion or lymph node metastases) declined from 13% to 6% [5]. Actuarial 10-year biochemical progression-free survival for organ-confined disease (stage pT2 with negative surgical margins) increased from 70% (pre-PSA era) to 86% (PSA era) [5].
Polascik et al. [11] analyzed 3,676 radical prostatectomy specimens procured between 1988 and 2006, assessing the clinical features associated with pathologic stage migration. The cohort was divided into three time periods: 957 cases (26.0%) comprising the “early PSA era” (1988–1995); 1,308 (35.6%) cases during the “transitional era” (1996–2000); and 1,411 cases (38.4%) in the “contemporary diagnostic era” (2001–2006). During the “early PSA era,” only 27 of 957 (2.8%) men had pT2a disease and 45 (4.7%) had pT2b disease that increased to 4.1% and 12.8% between 1996 and 2000, respectively (Fig. 4.2A). With respect to percent tumor involvement (PTI) and pathologic Gleason score (pGS), pT2a cases had minimal (PTI ≤ 5%) or small-volume (PTI 5.01–10.00) disease in 65% and 14% of cases, respectively, and were low grade (pGS ≤ 6) in 59% of cases during the three eras. Stage (pT2a vs. pT2b) significantly influenced PSA recurrence-free survival only in the contemporary era (2001–2006) (Fig. 4.2b) [11].
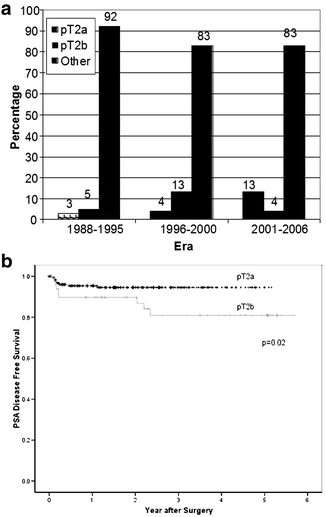

Fig. 4.2
pT stage distribution over three eras (a) with Kaplan–Meier PSA-free survival between pT2a and pT2b disease during 2001–2006 (b) (from Polascik et al. [11], with permission)
Pathologic stage is arguably the most accurate predictor of disease recurrence, more so than any of the pretreatment variables such as PSA, clinical stage, biopsy Gleason scores, or other such variables [11, 15, 21]. A consensus panel of the College of American Pathologists determined that pathologic TNM stage of PCa was the highest category I factor deemed to be of prognostic importance with utility in clinical patient management. For example, analysis of cancer control following radical prostatectomy in 1,000 consecutive patients treated at Baylor showed excellent biochemical disease-free survival (bDFS) (92.2%) over 14 years in patients with pT1–T2 N0 disease [22]. The high prevalence of early-stage disease, particularly in the United States and other well-screened European nations, opens the possibilities for AS or FT in appropriately selected patients and in a well-monitored setting to avoid overtreatment in men who might not otherwise need whole-gland therapy.
Tumor Volume to Determine Clinically Significant PCa
Tumor volume has also declined over the last 30 years from a mean of 4.7–6.1 cm3 to 2.1–2.6 cm3, respectively [21, 23]. Stamey et al. [4] first identified that tumor volume was linked to biochemical progression, occurring in 14% of men with cancer volumes ranging from 0.5 to 2.0 ml compared to 97% of men with tumor volumes >12 ml. The implication is that smaller tumor volumes are less often associated with progression and would therefore be more amenable to AS or parenchyma-preserving therapies in appropriately selected patients. Anast et al. [24] determined the mean tumor volume to be 0.23 ± 0.15 cm3 for insignificant and 2.36 ± 2.44 cm3 for significant cancers. In 55 (16%) of 336 patients in their RP series, Cheng et al. [25] identified an increased frequency of small-volume cancer (<0.5 ml). Renshaw [26] determined that patients with a tumor volume <1 ml did not have biochemical recurrence, while all patients with tumor volume >2 ml experienced recurrence. Eichelberger et al. [27] identified PSA recurrence in 15% compared to 73% of men with a maximal tumor diameter <1 and >2 cm, respectively.
Small-volume, clinically insignificant tumors on biopsy have recently been defined in a systematic review and meta-analysis as those with a single positive core, <3-mm cancer involvement, and GS ≤ 6 (no Gleason grades 4 or 5) [28]. The definition of clinically significant versus insignificant PCa is still debated. Based on prostatectomy specimens, a 0.5-cc cutoff volume (lesion of 10 mm in diameter) for a low-grade tumor (no Gleason grades 4/5) can be used to predict lifetime risk of mortality [29]. This issue of the index lesion and insignificant foci is still controversial as insignificant PCa micro-foci at RP have been associated with a comparable risk of biochemical progression as low-risk significant cancer [30]. In a study from the Mayo clinic between 1991 and 1993 for clinical stage T1c–T3 cancer, Dugan et al. reviewed 337 whole-mount RP specimens to understand what percent contained clinically insignificant cancer using four parameters such as grade, cancer volume, cancer volume doubling time, and patient life expectancy [31]. For cancer volume doubling time of 2, 3, 4, and 6 years, clinically insignificant cancer was identified in 1 (0.3%), 13 (3.9%), 25 (7.4%), and 49 (14.5%), respectively. Furthermore, a multicenter European study demonstrated that Epstein’s clinically insignificant PCa criteria underestimated true, clinically significant PCa in as many as one-fourth of patients [32].
Cancer volume likely represents an independent factor to predict cancer progression. AS is best suited for patients who have small-volume, insignificant cancer, while focal therapy should be considered for the patient with limited, clinically significant tumor. However, insufficient knowledge of individual tumor biology estimated by volume doubling time poses a great challenge in identifying candidates who may benefit from whole-gland compared to AS or parenchyma-sparing management options and renders accurate preoperative determination of clinically significant cancers questionable. There is not sufficient correlation between the amount of cancer reported in biopsies and that subsequently identified in RP specimens, especially for large cancers [33]. A greater percentage of tumors on needle biopsy is suggestive of large-volume, advanced-stage cancer, but the converse is often not true due to biopsy sampling limitations. Unifocal tumors have been associated with focal rather than multifocal high-grade prostatic intraepithelial neoplasia (PIN) [34]. Preoperative PSA and age did not differ between patients with two or more PCa foci compared to a single-focus lesion [34].
Approximately 80% of incidental autopsy and cystoprostatectomy-associated tumors are small volume (0.5 ml or less) with less than Gleason patterns 4 or 5, indicating that most secondary tumors identified preoperatively may not be of clinical significance [35]. Van Oort et al. [36] presented a single institution experience with cancer outcomes following RP for tumors that are of small volume (<0.5 cc) or deemed clinically insignificant (0.5 cc and GS < 7) based on pathology. The 5-year bDFS for both groups was 90% versus 35% in the rest of the cohort. Long-term clinical follow-up is warranted to determine the possible outcome differences among patients with small-volume PCa. Since the tumor volume of clinically significant lesion(s) has declined over time, it is now possible to consider AS or develop the concept of focal therapy to efficiently ablate only the small index lesion.
Number of Cancer Foci Identified in Prostatectomy Specimens
PCa has been believed to be a multifocal disease, and the treatment is predicated on whole-gland therapy. Given the stage migration toward early-stage disease, the question arises as to whether the number of cancer foci per prostate has similarly changed over time. It should be mentioned that studies cited in the literature likely differ in various methodologies that can influence the findings, including whether whole-mount pathology is used, how tissue is processed (slice thickness and intervening tissue between successive cuts), and how individual tumors are counted.
The mean number of PCa lesions identified in radical prostatectomy specimens during the pre- and early PSA era ranged from 5.2 (presumptive familial group) to 7.3 (sporadic group, with median 4) per prostate [37, 38]. In the early 1970s, more than 85% of all RP specimens demonstrated multifocal prostate cancer [38]. Most multifocal tumors encompassed small lesions, comprising 70% (presumptive familial) to 76% (sporadic) of all tumors [37, 38]. The number of tumors was broad, ranging from 1 to 60 cancers per prostate. Most contemporary cancers that include the large nodule type and small multifocal tumors are mainly located in the peripheral zone of the prostate, both in sporadic and presumptive familial groups. A study from Indiana did not identify significant differences in tumor location or multifocality when evaluating sporadic tumors and those with presumptive familial inheritance [25, 39].
The mean number of tumors per prostatectomy specimen has declined over time (Table 4.2) [37, 40–44]. A Baylor study reports that although tumor volume has decreased from the pre-PSA to the current PSA area, the number of cancers has slightly risen (Fig. 4.3) [41]. In a series of nearly 1,000 specimens, unifocal tumors were identified in 22% compared to 78% with multifocal disease, having a mean of 2.24 cancer foci per prostate (Fig. 4.4). Of the index tumor, the mean volume was 2.42 cc, and the mean total accessory tumor volume was 0.61 cc. The frequency of multifocal lesions increased from 71.5% (1983–1988) to 77.9% (1989–1993) to 80.8% (1994–1998) and was independent of patient age (Fig. 4.5). Alternatively, this may be due to a more thorough pathological assessment and increased awareness. Kaplan–Meier analysis of bDFS identified no significant differences in a subgroup analysis based on the number of foci present; however, a significant difference was identified between unifocal and multifocal disease (Fig. 4.6). The authors performed their analysis based on a zonal distribution (right peripheral zone, RPZ; left peripheral zone, LPZ; right transitional, RTZ; or left transitional zone, LTZ). Two separate foci were considered if there was an intervening whole-mount cut without tumor separating two foci in the same zone.
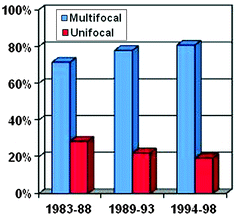
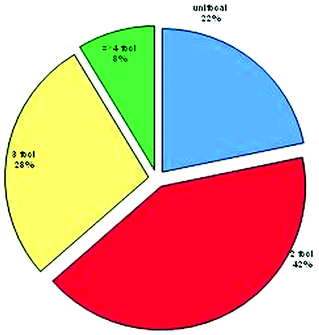
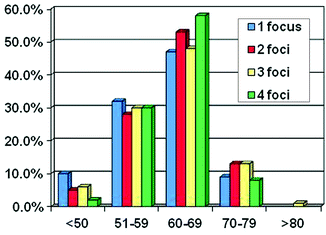
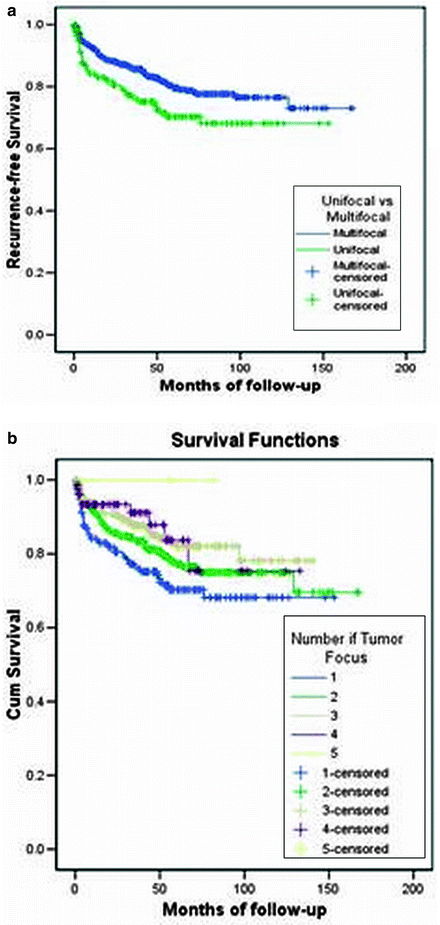
Table 4.2
Number of cancers per prostate: past and present
References | Year | No. of RP cases | Clinical stage | Pathological stage | No. distinct PCa foci (range) |
|---|---|---|---|---|---|
Bastacky et al. [37] | 1982–1991 | 81 | cT1–2 | pT2–3 | 7.3 (4.1–60)-Sporadic 5.2 (3.1–20)-Familial 6.8 (5.3–32)-Hereditary 6.0 (4.1–32)-Hereditary and familial |
Wise et al. [42] | 1992–1996 | 559 | cT1–2 | pT2–3 N0 M0 | 3.92 |
Muezzinoglu et al.[41] | 1983–1998 | 974 | cT1–2 | pT2–3 N0 M0 | 2.24 |
Yoon et al. [43] | 2005–2006 | 100 | cT1c–T2 (low-risk PCa) | pT2 | |
Ward et al. [44] | 1997–2006 | 180 | cT–2 | pT2–T3b | |
Haffner et al. [40] | 2005–2007 | 106 | cT1–2 | pT2–T3b | |
Nevoux (2011, personal communication) | 96 | cT1–2 | pT2 | 2.24 |

Fig. 4.3
Change in multifocality by time period

Fig. 4.4
Distribution of unifocal and multifocal tumors

Fig. 4.5
Age distribution in unifocal and multifocal tumors

Fig. 4.6
Kaplan–Meier curves for bDFS in unifocal versus multifocal disease. (a) Recurrence-free survival in unifocal versus multifocal tumors (p = 0.043). (b) Recurrence-free survival in tumors with different number of tumor foci (p > 0.05) [adapted from Wheeler (2009, personal communication)]
In a study of 100 RP specimens from patients with low-risk disease thought to have unilateral disease by routine office-based prostate biopsy, Yoon et al. [43] detected bilateral disease in 63% of cases, comprising on average 2.9 cancers (median 2.5, range 1–9) in the entire prostate.
Along with stage migration toward earlier disease, several reports suggest that the number of cancers per prostate has decreased. In contrast, other groups have demonstrated overall smaller cancer volume but an increased number of foci. With widespread PSA screening and efforts aimed at earlier detection, it is likely that smaller tumors will be detected, many of which may be multifocal. However, the biology of each of the individual foci will ultimately dictate the natural history of the disease.
Role of the Index Versus Satellite Tumor(s) in Cancer Progression
Is the index cancer responsible for tumor progression? Can secondary lesions threaten the patient’s well being? Should secondary lesions be taken into account in respect to treatment or chemoprevention, or solely the index tumors?
When a patient has several cancers located in the prostate, the index tumor is defined as the largest volume lesion and is presumed to be the main determinant for tumor progression and prognosis [27]. Unifocal cancer has been identified in 13–33% of RP specimens and is generally associated with lower grade, stage, and recurrence rates than multifocal cancers [21, 25, 43, 45].
Based on 222 men with T1c PCa treated with RP, Noguchi et al. studied the prognostic value of secondary cancers in men having multifocal, localized PCa [46]. The cohort was divided into three groups including those men with a single tumor (54 cases, 24%), an index tumor with secondary cancers <0.5 cc (86 cases, 39%), and an index tumor with secondary cancers >0.5 cc (82 cases, 37%). Among the three groups, the investigators did not detect any differences using multivariate analysis regarding preoperative PSA, number of positive cores, percent of Gleason grade 4/5 cancer in the needle biopsy, or histological features in RP specimens. However, when analyzing PSA failure rates among the 3 groups, the multifocal group with smaller secondary cancers had a better prognosis than the group with a single tumor [46]. This study suggests that patients with multifocal PCa do not necessarily have a worse prognosis and outcome than men with unifocal or unilateral lesions [46]. A report by Haffner et al. [40] studying 108 RP specimens suggested that secondary cancers can rarely be significant in volume. The association of two significant volume cancers >0.5 cc was identified in only 7% of cases (11 of 152 secondary cancers).
In a series of 486 men treated by RP, Wise et al. studied the behavior of small, independent cancers compared to the index lesion and its impact on biochemical disease-free survival (bDFS) [42]. Small tumor volumes averaged 0.63 cm3, while the mean index tumor volume was 4.16 cm3. The bDFS rates after RP were at least similar if not better for the index tumor group compared to the group with the index plus satellite cancers, although this difference became significant only for tumors ≥12 cc. This study suggests that the clinically important characteristic is the size and biology of the index lesion only.
Several reports contend that secondary small-volume tumors do not significantly influence the survival of patients after RP [21, 42, 46]. A study by Liu et al. revealed support for a single aggressive lesion affecting the mortality of patients with advanced PCa. A multi-institutional study using high-resolution genome-wide single nucleotide polymorphism and copy number survey reported on 94 anatomically separate cancer sites in 30 men who died from metastatic PCa [47]. These authors identified that lethal metastatic PCa had a monoclonal origin that maintained a unique genetic signature copy number pattern. These data in conjunction with a single-locus genetic study of advanced PCa evaluating the role of TMPRSS2-ETS in tumor progression demonstrated that in spite of common genetic heterogeneity in primary cancers, most metastatic cancers arise from a single clone [48].
Regarding the role of tumor volume (TV), Stamey et al. identified tumor volume to be an independent predictor of biochemical failure after RP [4]. Recently, the Stanford group assessed index tumor volume only, studying the predictive value of index tumor volume compared to total tumor volume [46]. Other studies do not implicate a role for tumor volume as an independent predictor of prognosis [21, 49].
In 865 RP patients with favorable clinical and pathologic characteristics, Guzzo et al. showed that minimal TV was associated with a diminished risk of biochemical failure [50]. The Duke group established that percent tumor involve-ment was a significant predictor of biochemical progression after RP for localized PCa [11, 51]. In a retrospective review of 739 patients who underwent RP with a median follow-up of 91.7 months, Chung et al. demonstrated that accurate determination of tumor volume, along with other accepted pathologic indices, is sufficient and preferred over percent cancer involvement for prognostication of PCa mortality [52]. Estimated TV might provide additional prognostic information for risk stratification, biochemical failure, and disease progression.
The effect and differences in pathological processing of the specimen need to be taken into consideration. At some institutions, whole-mount step processing is the norm, yet at others routine visual tumor volume estimate of the RP specimen is standard. Whole-mount pathology offers the ability to obtain precise, uninterrupted measurements of tumor volume at the cost of greater expense and time. In a study comparing both pathological processing methods, Hollenbeck et al. did not find a significant difference in the pathological outcome of 93 whole-mounted versus 554 step-sectioned cases [53]. It remains unclear as to the true effect each of the pathological processing methods has on study outcomes evaluating cancer volume and whether cancer(s) is the same tumor or separate adjacent foci within the prostate lattice.
The cancer volume of the index lesion may be considered the driving force of PCa progression and therefore should be identified and treated at an early stage. The majority of satellite lesions generally do not appear to be life threatening to the patient. Therefore, for patients considering FT, precise 3D mapping biopsies and/or imaging studies should be done to identify the index tumor and ensure that potential clinically significant small-sized lesions are not inadvertently missed.
Cancer Laterality and Unifocality
Unifocal and/or unilateral cancers would potentially allow image-guided targeted therapy or hemi-ablation, respectively, as a type of focal therapy. What is the prevalence of unilateral and/or unifocal cancer and the clinical ramifications regarding these clinical entities?
Conventional PCa treatment, namely, whole-gland therapies, dogmatically is founded upon the tenets of tumor heterogeneity and multifocality seen in 50–87% of cases [18, 21, 46, 54]. With prevalent PSA screening and early detection programs, investigators are now reporting an increased proportion of unifocal and/or unilateral disease (Table 4.3) [14, 15, 25, 37, 40–46, 54–63].
Table 4.3
Unifocality and unilaterality based on final pathological assessment of prostatectomy specimens
Study/group | No. of RP specimens | Risk/tumor features | Processing method | % | |
|---|---|---|---|---|---|
Unifocality | Bastacky et al./Johns Hopkins [37] | 27 sporadic 26 familial | All | 3-mm sections | 30 24 |
Arora et al./Indiana [55] | 115 | All | Whole mount | 13 | |
Muezzinoglu et al./Baylor [41] | 947 | All | Whole mount | 22 | |
Villers et al./Stanford [54] | 234 | All | 3-mm sections | 50 | |
Wise et al./Stanford [42] | 486 | All | 3-mm Sections | 17 | |
Noguchi et al./Stanford [46] | 222 | All | 3-mm sections | 24 | |
Rukstalis et al./MCP Haneman [56] | 112 | All | Whole mount | 21 | |
Boccon-Gibod/Paris [57] | 56 | All | 3-mm sections | 30 | |
Djavan et al./Vienna [45] | 308 | All | 4-mm sections | 33 | |
Horninger et al./Innsbruck [58] | 80 | PSA ≤3.25 ng/ml | Whole mount | 35 | |
Miller et al./Colorado [59] | 151 | All | Whole mount | 44 | |
Song et al./Seoul [60] | 132 | All | Whole mount | 67 | |
Ohori et al./MSKCC&Baylor [61] | 1,000 | Low-risk | Whole mount | 18 | |
Ward et al./MD Anderson, Houston [44] | 180 | All | 3-mm sections | 17 | |
Karavitakis et al./London [63] | 100 | All | Whole mount | 22 | |
Masterson et al./Indianapolis [65] | 1,374 | All | Whole mount | 13 | |
Bott et al./London [71] | 100 | All | Whole mount | 14 | |
Nevoux et al. (BJUI, 2011) | 96 | All | Whole mount | 42 | |
Unilaterality |