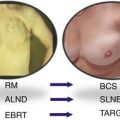
Inclusion criteria
Exclusion criteria
Age ≥45 yearsa
No informed consent
T1-2 (≤3.5 cma; mammography, ultrasound)
Non-compliance
cN0 (examination, ultrasound)
Age <45 yearsa
Histopathology: ductal-invasive
Tumour size >3.5 cm
Unifocal
cN+
Multifocal lesions
Lobular or non-invasive
Metastases
PBI should only be used in those patients who have an invasive ductal carcinoma (or other favourable subtype) as lobular carcinoma is more likely to present as multifocal and multicentric disease and as Anwar et al. (2010) found that almost 30 % of patients who underwent BCS for lobular carcinoma required further surgery as excised tumours had positive margins. All patients should undergo a triple assessment (mammography, ultrasound and histopathology) and the results should be discussed before selecting the patient for IORT. This allows for a more comprehensive view of the tumour in determining the appropriateness of the treatment for the patient.
While these criteria for patient selection are taken from the TARGIT-A trial, both the American Society for Radiation Oncology (ASTRO) (Smith et al. 2009) and the European Society for Therapeutic Radiology and Oncology (ESTRO) (Polgár et al. 2010) have released guidelines (Table 18.2) for the selection of patients for accelerated partial breast irradiation (APBI) outside the context of a clinical trial. It is important to note that these guidelines are based upon all trials and methods of APBI, of which IORT is a subset. They conclude that there appears to be a group of patients in whom the use of PBI could be deemed safe as the sole method of radiotherapy treatment but for some modalities there are limited recurrence and survival data.
Table 18.2
Selection criteria for PBI/APBI
Organisation/trial | Age (years) | Tumour size (cm) | Histopathology | Exclusion criteria |
|---|---|---|---|---|
ASTRO | ≥60 | ≤2 | Ductal invasive or other favourable subtypes | cN1, R+, L1, EIC, MF, HR negative |
ESTRO | >50 | ≤3 | Ductal invasive or other favourable subtypes | cN1, R+, L1, EIC, MF |
TARGIT-A | >45 | ≤2 | Ductal invasive | L1, R+, cN+, MF, EIC |
The TARGIT-A trial, which evaluates IORT as PBI, reported in 2010 (Vaidya et al. 2010) in The Lancet, low local recurrence rates (1 %) at a median follow-up of 4 years. These results are very encouraging though it is recognised that longer follow-up of these patients is needed.
Delegates at the 12th International Breast Cancer Conference in March 2011 were asked to vote on the use of IORT as a replacement for whole breast EBRT and the use of IORT as a replacement for the EBRT boost to the tumour bed. The vote was in favour of the use of IORT in both of these situations.
18.4 The Role of the Multidisciplinary Team
As previously stated, all patients should undergo a triple assessment to confirm the diagnosis of invasive breast cancer. The results of these investigations should then be discussed in a forum of clinical experts (e.g. breast surgeons, medical and clinical oncologists, radiologists, histopathologists). These experts work collaboratively and within their scope of practice in order to achieve the most appropriate decision regarding the best possible treatment for the patient. This forum also flags up patients who are suitable to enter the TARGIT-A trial, based upon the inclusion criteria. However, it is also required to justify the use of IORT outside the context of the trial as some patients may not fit the inclusion criteria but are deemed suitable for IORT when the guidelines from both ASTRO and ESTRO are taken into consideration.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree