13
Pediatric Brachytherapy
Lara Hathout, Suzanne L. Wolden, and Gil’ad N. Cohen
Historically, radiation therapy has played an important role in the treatment of pediatric malignancies especially for retinoblastoma (RB), neuroblastoma, brain tumors, and sarcomas (1). However, radiation therapy is associated with significant late side effects, such as growth and developmental failure, cardiac and pulmonary abnormalities, neurocognitive defects, infertility, and secondary cancers (2). With the advent of effective chemotherapeutic agents and enhanced surgical techniques, the use of radiotherapy in childhood cancers has declined over the past 20 years. The use of radiation therapy for acute lymphoblastic leukemia, non-Hodgkin lymphoma, and RB declined from 57%, 57%, and 30% in the 1970s to 11%, 15%, and 2%, respectively, in 2005 to 2008. In addition, a smaller decline has been observed in brain tumors (70% to 39%), Wilms’ tumor (75% to 53%), and neuroblastoma (60% to 25%) (3). Although external beam radiotherapy (EBRT) was the dominant form of radiation therapy, brachytherapy with or without combined EBRT increased in frequency in the mid-1980s for patients with brain tumors, soft-tissue cancers, and RB (3).
In order to limit long-term side effects, many trials have attempted radiation dose reduction such as in Hodgkin’s lymphoma (4–6), neuroblastoma (7–9), and Wilms’ tumor (10,11).
In addition, novel radiation therapies have emerged, such as intensity-modulated radiation therapy (IMRT) and proton beam therapy (PBT), with the main goal of achieving more conformal treatments while allowing sparing of normal organs. IMRT is now the standard technique for many cancers including pediatric tumors. However, there are several disadvantages including dose heterogeneity within the target volume, increased volume of normal tissue exposure, and beam leakage that may result in a significantly higher total body dose compared with conventional EBRT, which is nonnegligible in children given the significant risk of secondary malignancies and other late effects. Unlike photons, PBT deposits the dose in the “Bragg peak” over a relatively short distance with almost no exit dose resulting in effective sparing of critical structures. It is a very attractive treatment modality for pediatric malignancies; however, there are a number of relevant concerns: lack of clinical experience, known uncertainties in proton physics, and radiobiological effects (12). PBT is also very expensive and not available to many children worldwide.
The successful use of brachytherapy techniques in adults and the inherent tissue-sparing abilities of this modality continue to direct pediatric investigators to develop applications for their patients or to consider brachytherapy as a part of multimodality management.
The purpose of this chapter is to provide an overview of the role of brachytherapy in pediatrics. To achieve this goal, it is essential to understand the diagnoses amenable to brachytherapy in children, the number of potential cases, published experiences, potential complications, treatment objectives for pediatric patients, and concerns surrounding the use of this generally invasive, high-dose local control modality in children.
PEDIATRIC MALIGNANCIES
Pediatric cancers represent 1% of all new cancers diagnosed in the United States. According to the American Cancer Society (ACS) (13), an estimated 15,780 new cases and 1,960 cancer deaths are expected to occur among children and adolescents (aged 0–19 years) in 2014. In decreasing order of incidence, acute lymphocytic leukemia (26%), brain and central nervous system (CNS) (21%), neuroblastoma (7%), and non-Hodgkin lymphoma (6%) are the most common cancers in children aged 0 to 14 years. Among adolescents aged 15 to 19 years, Hodgkin lymphoma (15%), thyroid carcinoma (11%), brain and CNS (10%), and testicular germ cell tumors (8%) are the most common cancers (Figure 13.1). Although the incidence of childhood cancers have been slightly rising from 1975 to 2010 by an average of 0.6% per year (14), mortality rates declined steadily by an average of 2.1% per year based on the ACS Surveillance Research 2014.

Figure 13.1 Estimated cases of cancers among children aged 0 to 19 years with the observed 5-year overall survival. CNS, central nervous system.
Brachytherapy has been utilized as a salvage adjuvant therapy after prior surgical resection and even prior EBRT with some success. In this setting, local, regional, and distant failure is of concern depending on the tumor site and histology. For patients treated at diagnosis, particularly in children with sarcomas, brachytherapy is added as a high-dose adjuvant treatment to reduce the incidence of local failure. Oncologists often note that local control in many of these settings does not affect overall survival, as salvage surgery is available. Although this approach of radiation therapy avoidance is frequently utilized in the pediatric setting, both patients and caregivers should be educated about the relative risk of local disease recurrence in the absence of adjuvant radiation as well as the morbidity associated with a salvage surgical procedure. Moreover, brachytherapy is the most conformal radiation delivery treatment with minimal normal tissue irradiation resulting in a low risk of secondary malignancies compared with chemotherapy and EBRT.
Soft-Tissue Sarcomas
Soft-tissue sarcomas are classified into two categories: rhabdomyosarcoma (RMS), which represents 40% of soft-tissue sarcomas, and non-rhabdomyosarcoma (60%). Radical compartmental resection and amputation in soft-tissue sarcomas have been abandoned and replaced by limb-sparing wide surgical excision with satisfactory functional, cosmetic, and outcome results. Amputation is reserved to patients with high risk of limb-length discrepancies that may occur. The use of radiation therapy in soft-tissue sarcomas has been consistent since 1973 (3). Depending on histology, the use of radiation therapy with or without surgery provides excellent local control in the management of primary, recurrent, and metastatic sarcomas as reported in retrospective pediatric studies (15–18). In order to achieve higher local control rates, intraoperative brachytherapy has been used in combination with EBRT with promising results (19–23).
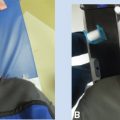
The most described high dose rate intraoperative radiation therapy (HDR-IORT) technique is the Memorial Sloan Kettering Cancer Center (MSKCC) technique using the Harrison–Anderson–Mick (HAM) applicator and an iridium-192 (192Ir) high dose rate afterloader (23–26). Following surgical resection, the operating surgeon and radiation oncologist identify the tumor bed. Normal tissues are moved out of the planned HDR-IORT field to the extent possible, with lead shielding used when needed to protect normal tissues. The applicator is placed on the tumor bed, secured with sutures if necessary, and firmly opposed to the treated surface with a surgical packing material (see Figure 13.2). Customized computer-generated graphically optimized plans are created for each patient based on the shape and total area treated, with dose prescribed to 0.5 cm depth from the surface of the applicators (24). Intraoperative treatment planning for HDR-IORT consists, usually, of an atlas-like plan where the treatment is rectangular and the dose to the prescription plane is uniform (Figure 13.3). Because the treatment area in pediatric cases is typically small and the curvature of the applicator is limited, the implant is assumed to be flat. The deviation associated with such an assumption is between +5% and −5% for concave and convex implant geometries, respectively. Often, there is a need to protect nearby critical structures. To complement physical retraction and placement of lead shields, the standard plan may require modification to further spare these structures. Such a modification may be achieved by lowering the prescription dose at one aspect of the implant, or disabling the source positions in that portion of the applicator altogether (27) (Figure 13.4). The dose delivered depends on the prior use of EBRT, the age of the patient, and the proximity of the normal tissues with a median dose of 12 Gy (range: 4–17.5 Gy). When postoperative radiation therapy is used, the EBRT dose can be lowered to 24 to 36 Gy instead of the 50.4 Gy necessary for definitive treatment (24). The results of this technique are encouraging with a 5-year local control rate of 63% and 5-year overall survival of 43%. Patients treated with HDR-IORT as part of the initial treatment had a significantly higher local control rate compared with those treated for recurrent disease (86% vs 46%). Acute and late grade 3 or greater toxicity occurred in 2.5% and 5.3% of patients 0.3 to 9.9 years, respectively, after HDR-IORT. The incidence of toxicity of grade 3 or greater level was not associated with HDR-IORT applicator size, HDR-IORT dose, prior RT or PORT, or prior or postoperative chemotherapy, but all toxicity of grade 3 or greater level occurred in patients who were younger than 6 years treated with HDR-IORT doses greater than or equal to 12 Gy. Therefore, it is recommended to use HDR-IORT doses of 8 to 12 Gy even for patients 6 years or younger.
Figure 13.2 HDR-IORT for recurrent intrathoracic neuroblastoma. The flexible HAM applicator is placed directly on the high-risk clinical target. The normal organs can be packed away from the applicator so as to shield dose. For immovable organs, customized lead shields can be interposed so as to minimize the dose. HAM, Harrison–Anderson–Mick; HDR-IORT, high dose rate intraoperative radiation therapy.

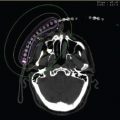
Figure 13.3 Standard rectangular IORT plan to deliver 12.5 Gy 5 mm from the surface of the HAM applicator (10 mm from the plane of the source). An atlas of common dimensions can be created so as to minimize planning time intraoperatively. Variations of these plans to allow for various degrees of curvature also improve the specificity of the dose for those situations. HAM, Harrison–Anderson–Mick; IORT, intraoperative radiation therapy.
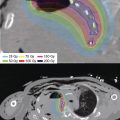
Figure 13.4 An IORT plan like the one in Figure 13.3 where source and prescription points have been disabled to spare nearby critical organs, while maintaining prescription dose at the remainder of the treatment plane. The shaping of the isodose distribution was effected by turning off four dwell positions in channel 1 and two in channel 2, shown at the top right of the image. IORT, intraoperative radiation therapy.
RMS of the vagina represents 3.6% of all RMS (28). The modern management of vaginal RMS involves a multimodality approach including neoadjuvant chemotherapy followed by local therapy translating in excellent outcomes. St. Jude Children’s Research Hospital reported its experience in the treatment of vaginal tumors in childhood; its findings confirmed the excellent prognosis of RMS arising in the vagina and the efficacy of a conservative approach to local therapy that leads to a high rate of cure (29). The Institute Gustave Roussy published its brachytherapy experience using manually loaded 192Ir wires to a total dose rate of 60 to 65 Gy delivered in one to three applications with acceptable normal organ mean doses with an overall survival rate of 80% at 10 years (30–32). Nag et al were the first to report HDR brachytherapy in children with RMS to a total dose of 36 Gy in 12 fractions (33). The role of radiation is essential; the local recurrence rate was significantly higher when radiation was omitted even in patients with a complete response after chemotherapy. Therefore, the international study, ARST0331, was amended for patients with group II or III vaginal RMS to include radiation therapy (34).
For the adult population, there is a wide range of prefabricated afterloading systems used for endocavitary vaginal brachytherapy; however, most of these applicators cannot be used in young patients. Custom-made vaginal cylinders dedicated to pediatrics with diameter sizes ranging from 1 to 2 cm exist (Figure 13.5). They offer many advantages: first, they are prefabricated and ready to use; second, they are fully dosimetrically characterized; and last, they have a single channel allowing simple radially symmetric dose distribution (35). However, it may be difficult to modify this dose distribution to spare critical structures and uninvolved healthy vaginal tissue without compromising the dose to the target. In order to increase treatment accuracy, daily positioning, and treatment reproducibility, a multichannel customized applicator can be used. This latter technique conforms better to the patient’s anatomy, and with verification imaging can be shown to be highly reproducible. The increase in degrees of freedom made possible by the introduction of multiple channels allows the sculpting of the dose distribution to provide adequate target coverage and possible sparing of the contralateral vaginal wall, and critical structures such as the bladder and rectum. CT or MRI treatment planning is necessary in order to properly account for the custom geometry of the applicator and the patients’ internal anatomy. While MRI offers superior visualization of organs and (if present) target, CT can be used to generate digitally reconstructed radiographs (DRRs) for use in daily positioning and treatment verification. An impression of the vaginal cavity can be performed in the operating room and an MRI-compatible mold can be created allowing MRI planning for vaginal RMS (35). A dose of 40 Gy in 10 daily fractions equivalent to 46.7 Gy equivalent dose per 2 Gy fraction (EQD2) for the tumor (α/β = 10) and 56 Gy for the normal tissues (α/β = 3) prescribed to the target volume is adequate (36). Doses of more than 5 to 10 Gy to the ovaries have been associated with ovarian failure (31); therefore, it is recommended to limit the dose to the ovaries to a mean dose less than or equal to 4 Gy.
Figure 13.5 Custom vaginal cylinders are shown (1.0 and 1.6 cm diameter) with a central tandem. A 2 cm-diameter adult cylinder is shown for reference. Note that the tandem is inserted to the tip of the cylinder (also called stump applicator).
The head and neck region is affected in 40% of RMS and divided into parameningeal, nonparameningeal, and orbital subsites. RMS of the orbit represents 10% of RMS (37). Generally, RMS of the orbit is associated with a good prognosis with a 5-year overall survival of 90% (37). Treatment consists of four to nine cycles of chemotherapy using a multidrug regimen. Radiation therapy is usually reserved for residual disease because it is associated with severe complications, such as cataract, retinopathy, opticopathy, dry eyes, growth disturbances of the irradiated bones, and brain damage (38). A study from the Netherlands reported the experience with intraoperative brachytherapy using a multichannel customized mold firmly applied over the at-risk surgical bed. A total dose of 40 to 50 Gy was prescribed to the clinical target volume (CTV) using either low dose rate or pulsed dose rate brachytherapy. Four out of 20 patients developed recurrence requiring exenteration, with a progression-free survival of 80% and a 5-year overall survival of 92%. Toxicity was relatively low, with ptosis, keratopathy, limited retinopathy, and cataract being the most common (39). Custom-mold HDR brachytherapy for sarcoma is applicable to many head and neck sites, and embryonal RMS of the soft palate (40), and a salvage technique involving exenteration and custom-mold brachytherapy has been used successfully for recurrent sarcoma of the orbit (41).
Retinoblastoma
The primary and secondary goals of RB therapy are cure and vision preservation, respectively. Enucleation is indicated in patients with unilateral RB and a blind eye or bilateral RB and both eyes are blind. Exenteration involves the removal of the globe, extra-ocular muscles, lids, nerves, and orbital fat. The indication of exenteration is extensive local tumor with globe infiltration and recurrence of tumor following enucleation.
Local therapy is now the mainstay of treatment for localized RB and includes cryotherapy, photocoagulation, laser hyperthermia, and radioactive plaque application.
Episcleral plaque brachytherapy has been used as the primary and salvage treatment of RB for decades. In the modern era of ophthalmic artery chemosurgery (OAC), few have reported the use of brachytherapy and OAC. OAC involves passing a catheter through the femoral artery and guiding it to the ostium of the ophthalmic artery, where chemotherapy is injected. It provides a high, localized concentration to the eye while limiting systemic exposure to cytotoxic drugs. Brachytherapy used as either adjuvant or salvage treatment following OAC is effective, even in the presence of vitreous seeding; the majority of eyes maintained stable or improved retinal function following treatment (42,43). The American Brachytherapy Society Ophthalmic Oncology Task Force (ABS-OOTF) recommends (Level 2 Consensus) that ideal tumors for primary brachytherapy are located anterior to the equator and, in unilaterally affected children, measuring less than 15 mm in base and no more than 10 mm in thickness (44). The ABS-OOTF recommends (Level 2 Consensus) that vitreous seeding should be absent or within 2 mm of the tumor surface. The most used radionuclides are iodine-125 (125I), palladium-103 (103Pd; low-energy photon-emitting sources), and β-emitting ruthenium-106 (106Ru). Each radionuclide offers a different energy, intraocular dose distribution, and requirements for handling (44). The dose for RB is 40 to 45 Gy prescribed to the tumor apex over 1 to 5 days. Before the procedure, the tumor’s maximal basal diameter and maximum height must be determined by ultrasonography. After a careful eye examination, the surgeon performs a peritomy, opens the conjunctiva, and snares the rectus muscles with either sutures or muscle hooks and rotates the eye. Afterward, the room is darkened and a transilluminator is placed over the pupil. The shadow cast by the tumor is marked on the sclera with a pen or electrocautery; tumors that cannot be transilluminated are visualized by ultrasound. An additional 2 to 3 mm margin is measured and marked around the tumor base. Some centers directly sew the plaque over the marked target, whereas others preplace sutures using “dummy” plaques.
Historically, 125I and 103Pd Collaborative Ocular Melanoma Study (COMS) plaque dose calculations for pediatric tumors have followed the more general guidelines of the COMS protocol. Dose calculations were made at the tumor apex, and assumed that it was at the plaques’ central axis. Under this formulation, a simplified one-dimensional TG-43 calculation was performed. Because the plaques are constructed such that the sources’ central axis points are orthogonal to the sources’ transverse axis, source anisotropy was ignored. Most clinical studies (for both adult and pediatric tumors) used this formalism for dose calculation, and accepted that the error in off-axis dose calculation exceeded 5%. More recently, Monte Carlo studies of the effects of plaque materials on the dosimetry revealed that, in addition to known uncertainties, the actual dose delivered could be less than 90% of the planned dose. This was shown to be a result of increased absorption in the silastic insert, as well as reduced scatter and absorption on the gold backing of the plaques. AAPM TG-129 (45) provides a detailed overview of dose calculations as well as guidance for implementation of the updated dose calculations. Figure 13.10 (located in Case 13.3) shows a typical dose distribution for a 16 mm COMS plaque with 125I seeds.
Another type of eye plaque using 106Ru, a β-emitter (half-life: 372 days, Emax: 3.54 MeV; Eavg: 1.42 MeV), is being widely used in Europe for the treatment of intraocular tumors. These plaques are thin (approximately 1 mm) compared with the COMS (~3 mm). Consequently, insertion and removal are much easier, especially for tumors located in the posterior aspect of the eye. Here, the plaque itself is the source, and the dosimetry of each plaque is fixed and is determined at the time of commissioning. Dosimetry for these treatments consists of calculation of treatment duration as a function of tumor depth, prescribed dose, and a correction for source decay. Because of the steep dose gradient associated with this β source, the treatment depth for these plaques has been limited to 6 mm from the surface of the plaque (Figure 13.9, see Case 13.3).
Neuroblastoma
Neuroblastoma accounts for 10% of pediatric cancers and is the second most common abdominal tumor after Wilms tumor. Most of these tumors occur in children less than 5 years of age. Surgery in neuroblastoma has both a diagnostic and therapeutic role. In children with localized disease and no evidence of metastases, complete gross excision can be achieved with cure rates as high as 90% in Stages I and II (46–48). For localized but unresectable tumors, chemotherapy and radiation can achieve adequate tumor regression enabling the surgery (49–51). Patients with high-risk neuroblastoma (age of more than 1 year, metastases, and MYCN oncogene amplification) have a poor prognosis with a long-term survival rate of less than 40% (52–54). Although external radiation is the primary modality for neuroblastoma given its high radiosensitivity and efficient local control rate (9,55), the role of IORT has been examined in a few studies with local control rates of 75% to 85% (56–58). In addition, IORT, to a median dose of 12 to 15 Gy using the HAM applicator, is a reasonable treatment for recurrent/persistent high-risk neuroblastoma with acceptable toxicity (59). This technique is similar to the HDR-IORT described earlier (Figure 13.2).
Craniopharyngioma
Limited surgical decompression followed by EBRT for residual tumor is the gold standard treatment of craniopharyngioma (60–63). Total resection is associated with a high incidence of postoperative diabetes insipidus (80%–90%) and morbid hypothalamic obesity (50%) (64–66). Given the cystic component of craniopharyngioma, intracavitary brachytherapy has been examined in several studies with reduction in the cysts in 70% to 100% of patients (67–70). A variety of β-emitting isotopes have been tested such as 32P, 90Y, and 186Re; 32P is the optimal isotope because it has a lower energy, longer half-life, and shorter half-value tissue penetrance than 90Y. Kinckingereder et al have published their experience with colloidal 32P intracavitary solution injection for cystic craniopharyngioma and reported a 5-year local control rate of 86% with minimal toxicity.
Using a sterile technique, the cyst cavity was accessed by the neurosurgery team. A predetermined amount of cystic fluid was removed before 32P was injected with the objective of returning the cyst to its original volume (or 30% of the original volume in symptomatic patients) in order to facilitate homogeneous 32P distribution around the surface of the cyst wall. The injection needles were barbotaged to ensure a homogeneous mixture of 32P and cystic fluid. Visual changes and endocrinological deterioration were mainly caused by tumor progression (71). A dose of 200 to 300 Gy is usually delivered to the cyst wall (71,72).
COMPLICATIONS AND SIDE EFFECTS OF BRACHYTHERAPY IN CHILDREN
Long-term complications of EBRT in the pediatric population on bone, soft tissue, normal organ function, and secondary malignancies are well documented (73–77). IORT is a very attractive treatment for children because it is highly conformal and improves local control with limiting late toxicity. A study from MSKCC on pediatric solid tumors reported a 12% (8 of 66 patients) rate of perioperative complications, mainly wound infections and intra-abdominal abscesses. Acute and subacute grade 3 and 4 complications occurred in three of eight patients, including a small bowel infarct, broncho-esophageal fistula, and hepatic veno-occlusive disease (25). Discriminating the cause of complications is difficult, especially in the setting of extensive surgeries, immunosuppressed patients, and previous EBRT. Rich et al reported an 18% rate of hydronephrosis requiring a stent, one case of bowel necrosis, one case of bowel perforation, and one case of anastomotic leak following IORT for patients with high-risk neuroblastoma (59). As for soft-tissue sarcomas, the toxicity rate after IORT is lower; acute and late grade 3 or greater level occurred in 2.5% and 5.3% of patients, respectively. The incidence of toxicity of grade 3 or higher was not associated with HDR-IORT applicator size, HDR-IORT dose, prior EBRT, or postoperative radiation (24) and was relatively similar to grade 2 or higher complications in the adult population (6%–30%) (78–81).
In the case of vaginal tumors in childhood, Spunt et al reviewed the late effects in female survivors of pelvic RMS, which included vaginal stenosis, fistulas, gastrointestinal strictures, and bladder dysfunction (82).
Complications following eye plaque for RB included cataracts in 10% to 43%, optic neuropathy in 2% to 42%, vitreous hemorrhage in 20% to 54%, and vitreoretinopathy in 10% to 59% of eyes (43,83–88).
Survivors of childhood cancers have a five-fold increase of secondary malignancies compared with the general population; however, the absolute risk is low: less than 1%. It should be mentioned that malignancies induced by radiation tend to be diagnosed at ages at which they would normally be diagnosed in the general population (89). Radiation-induced secondary malignancies generally occur within 4 to 10 cm of the field border. As for the cancers that occur outside the field, it could be explained by source leakage, collimator scatter, neutron productions by photonuclear interactions, and Compton scattering within the patient (90).