This article summarizes current state-of-the-art techniques used in the management of pediatric neurologic emergencies. Solutions to challenges faced by the radiologist, including the selection of an appropriate modality for an individual patient, are discussed. Imaging appearances of specific entities are described with an emphasis on conditions unique to the pediatric population.
This article summarizes current state-of-the-art techniques used in the management of pediatric neurologic emergencies. Solutions to challenges faced by the radiologist, including the selection of an appropriate modality for an individual patient, are discussed. Imaging appearances of specific entities are described with an emphasis on conditions unique to the pediatric population.
Special challenges in imaging the pediatric patient
Neuroimaging of the pediatric patient presenting to the emergency department is most often indicated for evaluating the child who is comatose or obtunded; has a change in neurologic status; presents with prolonged seizures; or suffers the neurologic consequences of a traumatic, infectious/inflammatory, vascular, or metabolic abnormality.
Among the challenges faced by the clinician and radiologist in this situation is the need to decide (1) whether imaging is required emergently, (2) which imaging modality is most appropriate for a particular clinical indication, and (3) whether the available imaging techniques must be modified to ensure a speedy and accurate diagnosis. In addition, there are certain characteristic findings on imaging studies in pediatric patients that deserve special mention.
This review aims to address the neuroimaging issues typically encountered in central nervous system (CNS) emergencies involving the pediatric patient, with illustrative examples and a discussion of the various imaging modalities.
How does emergency neuroimaging of the adult differ from that of the pediatric patient?
Whereas the adult brain is fully formed from a structural and functional standpoint, the pediatric brain (neonate to young adult) follows a steady and predictable course of development and maturation over the course of approximately 20 years, depending on the individual. Hence, the radiologist’s ability to perform and interpret neuroimaging studies in the infant, child, adolescent, and young adult hinges largely on knowledge of normal brain development, an understanding of the biologic and morphologic changes that take place from gestation through adolescence, familiarity with age-specific diagnoses, and a grasp of the specific normative appearances that characterize each stage of development.
It is also well known that the pediatric patient shows greater short- and long-term sensitivity to radiation than the adult patient. In particular, the potential for ionizing radiation (eg, that produced by radiographs and computed tomography [CT]) to harm children, especially those for whom serial imaging may be necessary, forms an especially important consideration when selecting the most appropriate imaging modality in the emergency department. Other factors in selecting the modality include the availability of equipment, the need for sedation or general anesthesia, and the clinical status of the child. For example, although magnetic resonance imaging (MRI) is ideally suited for evaluating a comatose child, CT or ultrasonography (US) may be considered adequate if the child is clinically unstable and would benefit from the rapid image acquisition and attendant diagnostic information generated by these modalities. When time and circumstances permit, the radiologist may decide to defer the study until the child is stabilized and a more definitive examination can be obtained.
Finally, except in instances of severe illness, cognitive impairment, or dementia, the adult patient is generally able to understand and cooperate with the goals of a neuroimaging study. In contrast, the pediatric patient’s ability in this regard depends on his or her developmental stage, on the amount of pain or distress caused by the pathology under investigation, and on the environment in which the study is undertaken.
These factors, separately and in combination, form the decision-making matrix for selecting the most appropriate neuroimaging modality for the pediatric patient in the setting of the emergency department.
How does emergency neuroimaging of the adult differ from that of the pediatric patient?
Whereas the adult brain is fully formed from a structural and functional standpoint, the pediatric brain (neonate to young adult) follows a steady and predictable course of development and maturation over the course of approximately 20 years, depending on the individual. Hence, the radiologist’s ability to perform and interpret neuroimaging studies in the infant, child, adolescent, and young adult hinges largely on knowledge of normal brain development, an understanding of the biologic and morphologic changes that take place from gestation through adolescence, familiarity with age-specific diagnoses, and a grasp of the specific normative appearances that characterize each stage of development.
It is also well known that the pediatric patient shows greater short- and long-term sensitivity to radiation than the adult patient. In particular, the potential for ionizing radiation (eg, that produced by radiographs and computed tomography [CT]) to harm children, especially those for whom serial imaging may be necessary, forms an especially important consideration when selecting the most appropriate imaging modality in the emergency department. Other factors in selecting the modality include the availability of equipment, the need for sedation or general anesthesia, and the clinical status of the child. For example, although magnetic resonance imaging (MRI) is ideally suited for evaluating a comatose child, CT or ultrasonography (US) may be considered adequate if the child is clinically unstable and would benefit from the rapid image acquisition and attendant diagnostic information generated by these modalities. When time and circumstances permit, the radiologist may decide to defer the study until the child is stabilized and a more definitive examination can be obtained.
Finally, except in instances of severe illness, cognitive impairment, or dementia, the adult patient is generally able to understand and cooperate with the goals of a neuroimaging study. In contrast, the pediatric patient’s ability in this regard depends on his or her developmental stage, on the amount of pain or distress caused by the pathology under investigation, and on the environment in which the study is undertaken.
These factors, separately and in combination, form the decision-making matrix for selecting the most appropriate neuroimaging modality for the pediatric patient in the setting of the emergency department.
Neuroimaging techniques
Plain Radiographs
Very few indications remain for performing plain radiographs to evaluate the neuraxis in the acutely ill child in the emergency department. Plain radiographs, however, continue to have a role in documenting fractures of the skull and appendicular skeleton in the suspected child abuse victim, but this is usually done after transfer from the emergency department and therefore will not be discussed further in this review. Plain radiographs remain the primary method for visualizing the entire cervical spine in children with traumatic injuries. In most children, anteroposterior and lateral spine radiographs suffice to clear the spine following trauma. The false negative rate for the single cross-table lateral view ranges between 21% and 26%. A 3-view series can improve sensitivity to 94% in children. More recent studies suggest that the open-mouth odontoid view is routinely beneficial only in children older than 5 years.
Some investigators have argued that multislice CT with sagittal and coronal reformats may be required in the obtunded patient to clear the cervical spine. At our institution, we reserve focused CT and MRI to clarify areas of abnormality and to define further injuries.
Ultrasound
Ultrasound (US) has numerous imaging advantages, including ready access, portability, real-time and multiplanar capabilities, and reproducible results. Moreover, it is a noninvasive, radiation-free procedure that can be performed at the bedside, in the intensive care unit, or on an intubated, ventilated baby following delivery. Ultrasonography is also useful in evaluating hydrocephalus, extra-axial fluid collections, and large intracranial hemorrhages in the young infant who is too ill to be transported to CT or MRI. Further, transcranial Doppler techniques have been used to correlate resistive indices with elevated intracranial pressure in patients with head trauma.
Computed Tomography
The widespread availability and speed of image acquisition has made CT the neuroimaging modality of choice in the acutely ill child over the past 2 decades. CT can detect acute intracranial hemorrhage, cerebral edema, hypoxic-ischemic injury, infarction, hydrocephalus/shunt dysfunction, neoplasm, or abnormal collections. In addition, modern multidetector CT scanners can acquire submillimeter-thick images, which can be manipulated to produce multiplanar reformats and 3-dimensional (3D) images, thereby facilitating rapid detection of skull and facial fractures. When performed with iodinated contrast media, CT provides information about both inflammatory and infectious lesions and their resultant complications.
CT angiography (CTA) provides accurate information for a variety of arterial and venous abnormalities in the acute setting. CTA also compares favorably with MR angiography (MRA) in evaluating vertebral artery dissection, as shown in a number of adult studies. CT venography (CTV) is the initial study of choice in many centers for assessing cerebral venous sinus thrombosis. Further, in evaluating the child presenting after major trauma, multidetector CT (MDCT) has shown great utility in imaging the head, cervical spine, chest, abdomen, pelvis, and, where applicable, the appendicular skeleton.
The primary disadvantage of CT is that it requires the use of ionizing radiation, which can have potentially harmful effects on the tissues of the pediatric patient, particularly if used for multiple studies over the course of weeks, months, or years. It is, therefore, imperative that alternatives to CT be considered in making the diagnosis. When CT is chosen as the imaging modality for a child, the radiologist should follow the ALARA (as low as reasonably acceptable) principle, which entails using appropriate age- and weight-based dose adjustment parameters available on modern scanners. In addition, the scan should be strictly limited to the area of concern.
Magnetic Resonance Imaging
The multiplanar capability of MR to show superior anatomic detail and tissue contrast without the harmful effects of ionizing radiation makes it the imaging modality of choice for evaluating the neuraxis in children of all ages presenting with acute neurologic symptoms in the emergency department. MRI is superior to CT in evaluating the posterior fossa and in detecting early cerebral edema and microhemorrhages. Further, MRI can provide functional and physiologic information about the brain that cannot be generated by other modalities. Use of higher field strengths (most commonly 3-Tesla MRI scanners) has also demonstrated improved diagnostic accuracy for many conditions affecting the CNS.
Advanced MR imaging sequences, including susceptibility-weighted imaging (SWI), diffusion-weighted and diffusion-tensor imaging (DWI and DTI), magnetic resonance spectroscopy (MRS), and perfusion imaging, including arterial spin labeling (ASL), are being increasingly incorporated into acute pediatric neuroimaging protocols.
Susceptibility-weighted imaging
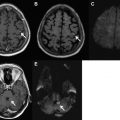
SWI imaging uses the paramagnetic property of blood products and increases visibility of microhemorrhages by accentuating signal dropout by rapid spin dephasing. It has been shown to be more sensitive than conventional MRI in detecting intraparenchymal blood products after traumatic brain injury in children ( Fig. 1 ). The technical aspects of SWI and its role in the detection of venous thrombosis, intracerebral hemorrhage, and vascular malformations have been described in a number of original studies and reviews. SWI has several advantages over conventional gradient-echo sequences, including its potential ability to differentiate between hemorrhage and calcium, as well as its ability to visualize vessel connectivity and microbleed location in relation to the vasculature and other structures in the brain. Methods for evaluating the arterial and venous systems simultaneously at higher field strengths (3 T and higher) using postcontrast SWI sequences have also been described. By optimizing the flip angle and echo times, the arterial and venous circulations can be separately visualized on maximum and minimum intensity projections. The value of this method has not yet been fully evaluated in children.
Diffusion-weighted imaging and diffusion tensor imaging
DWI is being increasingly used in the acutely ill child for early detection of ischemia, differentiating between infectious and inflammatory collections, and predicting of outcome in traumatic brain injury (TBI). The most common cause of decreased diffusion is ischemia, although it is important to remember that decreased diffusion can be seen in cellular tumors, abscesses, encephalitis, TBI, edema caused by astrocyte swelling in metabolic disorders, and in areas of intracerebral hemorrhage. The main advantage of DWI in acute brain injury is that the decrease in apparent diffusion coefficient (ADC) can be appreciated within minutes to hours following the insult. This information is vital to planning therapies for acute stroke and for initiating interventions that are neuroprotective, eg, hypothermia induction in hypoxic-ischemic injury and severe TBI.
DTI provides visualization of fiber bundle direction and integrity with in vivo characterization of the rate and direction of white matter diffusion. In the normal brain, water diffusion is restricted to the axis parallel in the direction of the axons. This property is termed “anisotropy.” The proportion of the diffusion tensor that is attributable to anisotropy is termed “fractional anisotropy” (FA). A decrease in FA in the setting of trauma has been shown to indicate white matter damage. By placing regions of interest over specific tracts, valuable information can be gained about the disruption caused to various white matter networks in the setting of acute brain injury. The role of DTI in defining the diagnosis, management, and prognosis of children with acute brain injury is being currently investigated. Early results indicate a significant decrease of FA in young children with TBI in the genu of the corpus callosum and internal capsules, and that differences in white matter integrity in children after early TBI may persist for several years.
Perfusion imaging
MR perfusion imaging is a technique used to evaluate cerebral perfusion dynamics by analyzing hemodynamic parameters including relative cerebral blood volume, relative cerebral blood flow (CBF), and transit time. It can be performed with a T2*-weighted dynamic susceptibility technique, ASL, or a T1-weighted dynamic contrast-enhanced perfusion technique. We reserve the use of perfusion imaging in the emergency setting at present to the evaluation of new tumors. The feasibility and utility of ASL perfusion MRI for characterizing alterations of CBF in pediatric patients with arterial stroke and other neurologic emergencies are being actively investigated. The short acquisition time, lack of ionizing radiation, widespread availability of MRI scanners, and ability to detect small regional hemodynamic alterations make perfusion techniques a potentially attractive technique to consider in the critically ill neonate.
Magnetic resonance spectroscopy
MRS has been used as a noninvasive in vivo technique that measures concentrations of various compounds within a sampled region in the brain to provide metabolic diagnostic indices beyond anatomic information. Its role has been documented in acute ischemic injury, metabolic disorders, and differentiation between abscesses and tumors. An early report indicated that MRS can have a role in differentiating between tumefactive demyelination and neoplastic lesions. More recently, it has been suggested that MRS shows some promise as a quantitative outcome-prediction tool in the subacute phase of TBI. The utility of MRS in making prognoses has not yet been established sufficiently to advocate its use in all routine MRI brain studies performed in the acutely injured pediatric patient.
Magnetic Resonance Angiography and Magnetic Resonance Venography
Magnetic resonance angiography (MRA) has shown considerable utility in the emergency setting for assessing aneurysms and arteriovenous malformations in patients presenting with intracranial hemorrhage, and in identifying dissections of the vertebral and internal carotid arteries following trauma. Recent studies have shown that, in some cases, 2D time-of-flight MRA may not be as sensitive as conventional angiography for detecting vertebral or internal carotid artery dissection in children; however, it is used successfully as an initial screening tool.
MRI with magnetic resonance venography (MRV) is the imaging study of choice in older children being evaluated for dural venous sinus thrombosis owing to their relatively clinically stable condition and their tolerance for longer imaging times compared with infants and toddlers. In addition, MRI/MRV can characterize associated parenchymal lesions without the use of ionizing radiation. One potential pitfall of MRV in neonates, however, is the increased flow gaps in the venous sinuses, particularly affecting the posterior aspect of the superior sagittal sinus, which can be attributed to the age-related smaller caliber of the sinus, smaller venous flow, and skull molding. At our institution, postcontrast, 3D, fast spoiled gradient-echo images (3D-FSPGR) have proved especially useful in cases where MRV is equivocal, or is affected by slow flow, in veins proximal to thrombus.
Etiopathology of neurologic emergencies in a child
The differential diagnoses in the child considered for emergent neuroimaging can be roughly divided into the following groups, as shown in Table 1 . These groups are specific to (1) the pediatric patient, (2) the imaging features of the suspected or known condition, and (3) the diagnostic algorithms that are selected in performing the examination. The pediatric patient differs significantly from the adult patient who may present with a similar pathology, and these differences are presented in the remainder of this article.
| Acute Hydrocephalus |
| Trauma |
| Accidental |
| Nonaccidental |
| Non-traumatic vascular events |
| Arterial ischemic stroke |
| Venous thrombosis |
| Embolic stroke |
| Vasculitis |
| Dissection |
| Migraine |
| Hypoxic-ischemic injury |
| Strangulation |
| Cardiorespiratory insufficiency |
| Near-drowning |
| Electrolyte and hormonal imbalances |
| Hypoglycemia |
| Hyponatremia |
| Hypocalcemia |
| First-time seizure and status epilepticus |
| Infection |
| Congenital |
| Acquired |
| Complications and sequelae |
| Demyelination |
| Acute disseminated encephalomyelitis (ADEM) |
| Multiple sclerosis (MS) |
| Metabolic disease (acute presentation) |
| Iatrogenic, toxic and drug-related injury |
| L-asparaginase |
| Cyclosporine |
| Methotrexate |
| Recreational drugs |
| Carbon monoxide |
| Neoplastic disease (acute presentation) |
| Encephalopathy in hematologic and oncologic disease |
| Posterior reversible encephalopathy (PRES) |
| Posttransplant lymphoproliferative disorder (PTLD) |
Hydrocephalus
Acute Hydrocephalus
Hydrocephalus can be caused by either overproduction of cerebrospinal fluid (CSF) by a tumor such as choroid plexus papilloma, by an obstruction to normal CSF flow, or by decreased CSF absorption. The latter 2 problems result in “obstructive hydrocephalus.” Obstructive hydrocephalus can be further divided into communicating hydrocephalus, where there is obstruction to CSF flow or diminished CSF absorption, and noncommunicating hydrocephalus, where there is intraventricular obstruction to CSF flow. More recent classifications that are based on the effect of pressure-flow derangements in CSF dynamics within the circuit diagram of CSF flow are designed to serve as a template for the study, nomenclature, and treatment of hydrocephalus.
In the emergency setting, there are 2 common scenarios that often warrant imaging: (1) the child with shunted hydrocephalus, and (2) the acute presentation of noncommunicating hydrocephalus.
Child with Shunted Hydrocephalus
The child with known shunted hydrocephalus can present with nausea, vomiting, irritability, fever, altered level of consciousness, or increased seizure frequency. Papilledema, cranial nerve palsies, hyperactive reflexes, and ataxic gait may be found on examination. Infants may present with increased head circumference, a bulging fontanelle, or splayed cranial sutures. Neurosurgical consultation is mandatory if shunt malfunction is clinically suspected.
Ultrasound can be considered in the young infant with large, open fontanelles in this setting. However, in most cases, CT is the modality of choice because of its ready availability, ability to detect changes in ventricular size and configuration, easy identification of intracranial hemorrhage or infarction, shunt catheter discontinuity in the skull and upper neck, and catheter migration.
A low-dose CT technique can be used to minimize radiation risks in these patients, especially those in whom multiple CT scans are required over their life span. Several authors have shown that there is no significant loss in diagnostic accuracy when such techniques are used in this clinical setting.
At our institution, a limited axial T2-weighted MRI in an imaging plane similar to that used in prior CT studies is used. This approach offers an attractive imaging alternative for the stable, older child who can lie still for a few minutes in the MR scanner. With the increased availability of MRI, and the advent of faster pulse sequences and motion correction software, hydrocephalus may also be safely and reliably assessed in younger patients without the need for sedation, while being spared the potentially harmful effects of ionizing radiation.
When evaluating a CT in a child with hydrocephalus, comparison with a baseline study obtained after successful shunt placement and serial prior scans can help detect subtle evidence of shunt malfunction. A single shunt may fail in patients with multiple shunts, and comparison with prior studies is essential to determine which shunt is malfunctioning. Ultrasound or MRI examinations performed since the prior CT must be reviewed to avoid errors that result from a failure to recognize changes that may have occurred in the interval between the 2 CTs.
In the patients with suspected shunt malfunction, a plain radiograph shunt series is often performed to differentiate between obstruction and other causes of mechanical shunt malfunction including catheter fracture. It is important not to mistake the radiolucent areas between the intermittent radiopaque markers seen in some shunts for areas of discontinuity. Shunt mechanism disconnection occurs most commonly at the level of the valve where the proximal and distal tubing meet. The actual fracture of the catheter tubing, however, usually occurs in the neck, likely resulting from its increased mobility.
Abdominal ultrasound and/or CT may be useful in determining the presence of ascites or an intra-abdominal mass such as a large CSF pseudocyst. In some cases, shunt malfunction may not always cause absolute or relative ventriculomegaly. CT scans performed in children experiencing true shunt malfunction may be normal, have stable ventriculomegaly, or even show decreased ventricular size compared with prior studies. Neurosurgical consultation is mandatory if shunt malfunction is clinically suspected, despite normal imaging.
Acute Presentation of Noncommunicating Hydrocephalus
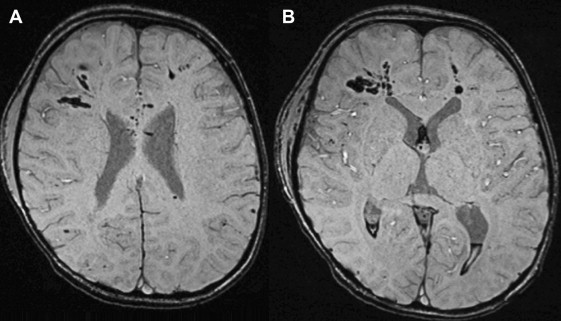
Noncommunicating hydrocephalus is caused by intraventricular obstruction of CSF flow. Obstruction sites are most commonly located where the CSF pathway is narrowest, namely at the foramen of Monro, cerebral aqueduct, and fourth ventricle and its outflow foramina. In the child, tumors are a common cause of such obstruction.
Tumors obstructing the foramina of Monro can involve the lateral ventricles (eg, choroid plexus tumors, astrocytomas, and ependymomas) or extend anteriorly from the third ventricle (eg, astrocytomas, choroid plexus tumors, and craniopharyngiomas) ( Fig. 2 ). Similarly, third ventricle tumors can extend posteriorly and inferiorly to obstruct the cerebral aqueduct. Most commonly, aqueductal obstruction is the result of a pineal region tumor including pineal origin tumors; germ cell tumors; astrocytomas arising from the tectal plate, thalamus, or quadrigeminal plate; or tentorial meningiomas and vein of Galen malformations. In patients with tuberous sclerosis, subependymal giant cell tumors originating in the region of the foramen of Monro can grow to obstruct the lateral ventricles.
Posterior fossa tumors in the pediatric population include the medulloblastoma, pilocytic astrocytoma, ependymoma, and, occasionally, cerebellar hemangioblastoma. Lesions of this type can cause fourth ventricle or aqueductal obstruction either by tumor extension into the ventricle, or by extrinsic compression of the ventricular walls.
MRI is the imaging modality of choice in these cases, although CT can identify many of these lesions as an initial screen in the emergency room and can demonstrate hydrocephalus. Though rare, primary and secondary (leptomeningeal metastases) tumors in the spine and spinal cord can cause hydrocephalus. The spine should therefore be imaged in all cases of new-onset unexplained hydrocephalus.
Non-neoplastic causes of noncommunicating hydrocephalus include arachnoid cysts, aqueductal stenosis, aqueductal webs, and congenital anomalies such as Chiari malformations. A discussion of these entities is beyond the scope of this article. In our experience, the 3D FIESTA (fast-imaging employing steady-state acquisition) sequence can play a useful role in evaluating new-onset hydrocephalus. This technique enables detection of arachnoid cysts and septations within the ventricles, and it can help confirm patency of the aqueduct. In some cases of Chiari 1 malformation, a phase contrast, velocity-encoded CSF flow study may also demonstrate CSF flow aberrations at the foramen magnum, eg, decreased flow posterior to the cerebellar tonsils.
Communicating Hydrocephalus (Extraventricular Obstruction to CSF Flow)
Extraventricular obstruction to CSF may result from intracranial hemorrhage, bacterial or granulomatous meningitis, CSF seeding of tumor, venous hypertension, or normal pressure hydrocephalus. Some of these entities are discussed in other parts of this article and in other articles in this issue: “Hemorrhagic Stroke and Nontraumatic Intracranial Hemorrhage,” “Central Nervous System Infections,” and “Intracranial Hypo- and Hypertension.”
Nonaccidental trauma/child abuse
Child abuse is a global problem, with head trauma being the leading cause of morbidity and mortality in abused children younger than 2 years. Nonaccidental injury is a leading cause of brain injury in infants, and is associated with high morbidity (up to 45%) and mortality (6%–26%). Nonaccidental injury should be suspected in the infant or young child who presents with the following :
- •
When there is no history of injury
- •
When there is a discrepancy between the explanation and nature of lesions
- •
When there are injuries of various ages
- •
When there are multiple injuries
- •
When there are associated retinal hemorrhages
- •
When there is a change or inconsistency in the history, delay in medical care, repeated injuries, or overall poor care.
The initial presentation varies from an impaired level of consciousness with significant neurologic damage to a child with relatively minor symptoms. The neuroimaging findings in these patients also vary, ranging from skull fractures to extra-axial hemorrhages, with or without concomitant brain parenchymal injury.
The decision to perform acute neuroimaging in the child who presents in the emergency department with suspected abuse is difficult at best. Clinicians agree that neuroimaging must be performed in all children with overt neurologic symptoms, but for those without obvious CNS involvement, the decision is less clear. Some investigators suggest, however, that even children without apparent neurologic signs or symptoms, but who present with other signs of physical abuse, may have intracranial injury and benefit from neuroimaging.
Currently, CT is the recommended initial imaging modality for detection of acute blood and fractures in pediatric patients suspected of abuse. Multiplanar reformatting of MDCT images can help identify small extra-axial and parenchymal contusions. Further, 3D reformats can also increase the ability of CT to detect skull fractures and differentiate fractures from normal variants.
Recent literature suggests that MRI (including DWI, MRS, and T2* and/or SWI imaging) should promptly follow the initial CT. MRI is the modality of choice for determining the sequelae of injury, which can include small extra-axial hemorrhages, brain contusions, shear injury, and infarction that may be difficult to identify on CT. Indeed, additional significant or evolving abnormalities are found on MRI in up to 25% of patients with an abnormal early CT examination. The SWI sequence (described earlier in this article) obtained on higher field strength magnets has also increased the sensitivity of MRI for the detection and differentiation of subarachnoid blood from extra-axial blood.
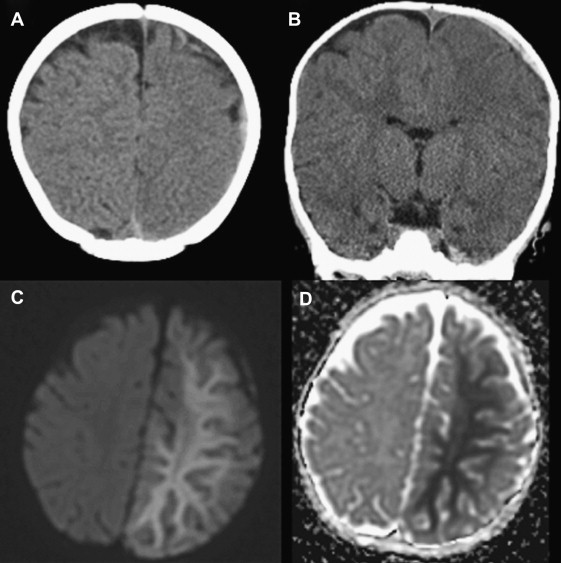
DWI is invaluable in this scenario, as it identifies hypoxic–ischemic injury in the first few hours after the trauma, and it is more accurate and sensitive in detecting hypoxic injury earlier than CT. MRI with diffusion has demonstrated increased sensitivity in the early detection of injury in children with nonaccidental head trauma. In addition to the well-described findings of extra-axial hemorrhage, global or focal ischemia, and skull fractures in these infants, more recent findings that support inflicted trauma include a small subdural hematoma with a disproportionately larger area of underlying unilateral hemispheric white matter edema ( Fig. 3 ). One group has suggested that this may be a sign of head injury related to abuse, arising specifically from cervical vascular compression, whether from kinking during hyperflexion/hyperextension, or from direct strangulation. Follow-up imaging in these cases demonstrated extensive cortical infarction. The role of MRI/DWI in children with a normal early CT, and the optimal timing of MRI/DWI after early CT in patients with suspected nonaccidental trauma, have not yet been fully determined, however.