, Mark J. Jameson2, David C. ShonkaJr.3, Max Wintermark4 and Sugoto Mukherjee5
(1)
Division of Neuroradiology, Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, USA
(2)
Division of Head and Neck Surgical Oncology, Department of Otolaryngology – Head and Neck Surgery, University of Virginia Health System, Charlottesville, VA, USA
(3)
Division of Head and Neck Surgical Oncology, Department of Otolaryngology – Head and Neck Surgery, University of Virginia Health System, Charlottesville, VA, USA
(4)
Department of Radiology and Medical Imaging, University of Virginia Health System, Charlottesville, VA, USA
(5)
Division of Neuroradiology, Department of Radiology and Medical Imaging, University of Virginia Health System, Charlottesville, VA, USA
Abstract
The structures of the face are formed from five mesodermal processes: the midline frontonasal process and the paired maxillary and mandibular processes (Figs. 13.1 and 13.2).
13.1 Embryology: Key Concepts
The structures of the face are formed from five mesodermal processes: the midline frontonasal process and the paired maxillary and mandibular processes (Figs. 13.1 and 13.2).
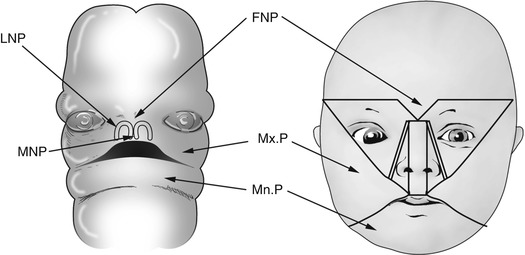
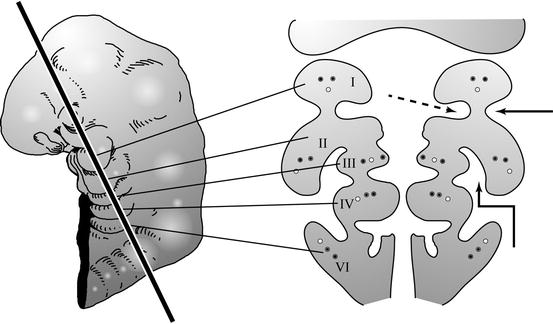

Fig. 13.1
Development of the face. FNP frontonasal process, Mx.P maxillary process, Mn.P mandibular process, LNP lateral nasal process, MNP medial nasal process

Fig. 13.2
Coronal section through the branchial arches. The branchial arches are paired mesodermal structures in the fetal neck, each with its own artery, vein, and nerve. The straight arrow points to a branchial cleft and the dashed arrow to a branchial pouch. The clefts are lined by ectoderm and the pouches by endoderm. The bent arrow points to the sinus of His, a cleft between the overgrown second arch and the external surface of the branchial apparatus. Second branchial anomalies are believed by some to arise from persistence of remnants of the sinus of His
The nose is formed by the union of two nasal placodes, each comprised of a nasal pit surrounded by a medial and lateral nasal eminence. The medial nasal eminences unite to form the septum, primary palate, and philtrum. The lateral nasal eminences form the lateral nasal walls and nasal ala. These are separated from the maxillary processes by the nasolacrimal groove, within which the nasolacrimal duct develops as a solid cord of cells that later canalizes.
The nasal cavity is separated from the oral cavity by the oronasal membrane.
The maxillary processes give rise to the zygoma, maxilla, and hard palate.
The developing nasal bones are separated from the frontal bone by a transient fontanelle – the frontonasal fonticulus. The nasal bones are separated from the underlying cartilaginous nasal capsule by the prenasal space, which transmits a dural diverticulum. This diverticulum exits the cranial cavity through the foramen cecum in the midline anterior skull base and extends to the tip of the nose. Both the fontanelle and the diverticulum are obliterated by growth of the frontal and nasal bones.
The branchial arches are five bars of mesenchyme (numbered 1 to 4 and 6) in the developing neck. Each arch contains a nerve, artery, and vein. The arches are separated on their external aspect by ectoderm-lined clefts and on their internal (luminal) aspect by endoderm-lined pouches. The derivatives of the branchial arches are indicated in Box 13.1.
13.1.1 Box 13.1. Derivatives of the Branchial Arches
Branchial arch | Nerve | Muscles | Ligaments | Bones | Pouch derivative | Cleft derivative |
|---|---|---|---|---|---|---|
I | Trigeminal | Muscles of mastication, tensor palati, anterior belly of digastric, mylohyoid | Lateral ligament of the malleus | Malleus, incus | Eustachian tube, middle ear cavity | External auditory canal |
II | Facial | Muscles of facial expression, stylohyoid, posterior belly of digastric, stapedius | Stylohyoid ligament | Stapes, lesser horn of the hyoid, styloid process | Faucial tonsillar crypts | |
III | Glossopharyngeal | Stylopharyngeus | Greater horn of the hyoid | Inferior parathyroid gland, thymus | ||
IV | Vagus | Laryngeal and pharyngeal musculature, except the stylopharyngeus and tensor palati | Superior parathyroid gland, C cells of the thyroid | |||
V | Spinal accessory | Trapezius, sternomastoid |
13.2 Congenital Anomalies of the Nose and Nasal Cavity
The three most important developmental nasal abnormalities that result in respiratory distress at birth are choanal atresia, congenital piriform aperture stenosis, and congenital nasolacrimal duct mucoceles. These all cause nasal obstruction and are symptomatic because infants are obligate nasal breathers at rest. Midline nasal anomalies include nasal dermal sinuses, dermoid and epidermoid cysts, nasal gliomas, and encephaloceles.
13.2.1 Choanal Atresia
Choanal atresia (Fig. 13.3) results from persistence of the primitive oronasal membrane that separates the nasal cavity from the oral cavity, either unilaterally or bilaterally. When choanal atresia is bilateral, it is symptomatic at birth. In the absence of symptoms, choanal atresia is suspected when the pediatrician is unable to pass a nasogastric tube. The atresia may be osseous, membranous, or osteomembranous and is best evaluated by CT. The most common finding on imaging is thickening of the vomer. It is important to obtain axial images of the nasal cavity in a plane parallel to the hard palate; if this is not done, the posterior choanae may appear artifactually narrowed. It is also important to suction secretions from the nasal cavity prior to imaging in order to be able to assess the cause of atresia accurately. The orbits and temporal bones should be evaluated for coexisting anomalies that may indicate the CHARGE association (coloboma of the eye, heart defects, atresia of the choanae, retardation of growth and/or development, genital and/or urinary abnormalities, and ear abnormalities/deafness). Imaging features of the CHARGE association are discussed in the chapter on the temporal bone and skull base.
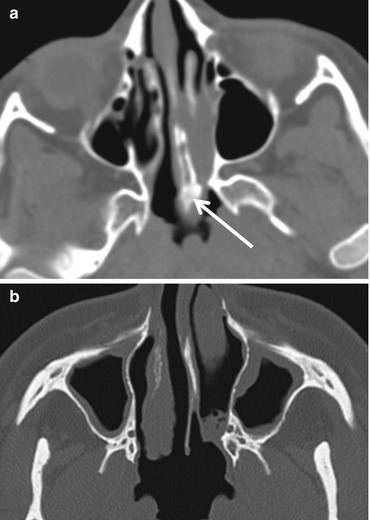

Fig. 13.3
Choanal atresia. (a) Osteomembranous atresia. Note the thickened vomer (arrow). (b) Membranous atresia
13.2.2 Congenital Piriform Aperture Stenosis
Piriform aperture stenosis (Fig. 13.4) arises from abnormal development of the medial nasal eminences which results in failure of formation of the primary palate. The piriform aperture of the nasal cavity is therefore stenotic and the palate is narrow. It may be recognized clinically by a complete inability to pass a nasal catheter. This abnormality may be associated with fusion of the central maxillary incisors, forming a single central or mega incisor. Midline abnormalities of the brain including corpus callosal agenesis and holoprosencephaly may coexist. The stenotic piriform aperture is best evaluated with CT; it is useful to obtain an MRI to evaluate for coexisting brain abnormalities.
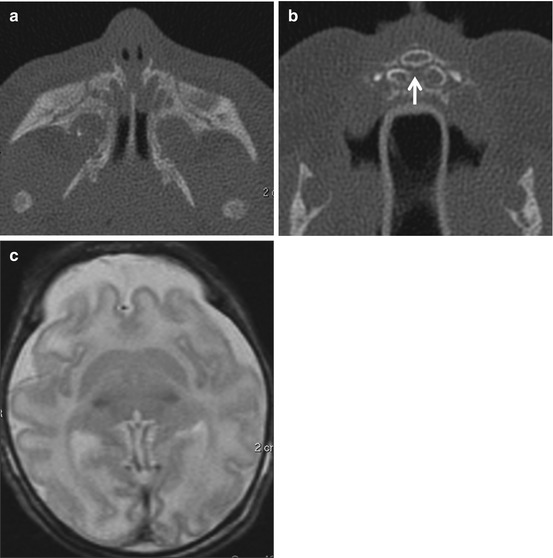

Fig. 13.4
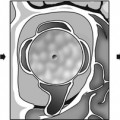
Congenital piriform aperture stenosis. (a) The nasal processes of the maxillae approximate each other closely to narrow the piriform aperture. CPAS is considered to be present if the width of each PA is less than 3 mm or if the total width is less than 8 mm. The presence of a single unerupted maxillary megaincisor (b) should prompt evaluation for intracranial anomalies (holoprosencephaly – note the forebrain fusion in (c) (Images courtesy of C. Douglas Phillips, MD)
13.2.3 Nasolacrimal Duct Mucoceles
These mucoceles (Fig. 13.5) result from obstruction of the nasolacrimal duct by an imperforate valve of Hasner, a membrane that guards the inferior opening of the nasolacrimal duct. Normally, perforation of the valve of Hasner occurs immediately after birth due to increase in pressure within the lacrimal system when the infant cries. If perforation does not occur, a mucocele results. The process may be bilateral, and when the mucoceles are large enough, they may encroach upon the nasal cavity to produce respiratory distress. The lacrimal sac may also be dilated, especially if the valve of Rosenmuller, a membrane that is normally present at the junction of the common lacrimal canaliculus and the sac, is also imperforate. These are best evaluated by unenhanced CT. The most important differential diagnoses for an intranasal mass in a neonate are encephaloceles, dermoids, teratomas, and hemangiomas. The characteristic low density of the mucocele and its location along the course of the nasolacrimal duct are usually diagnostic.
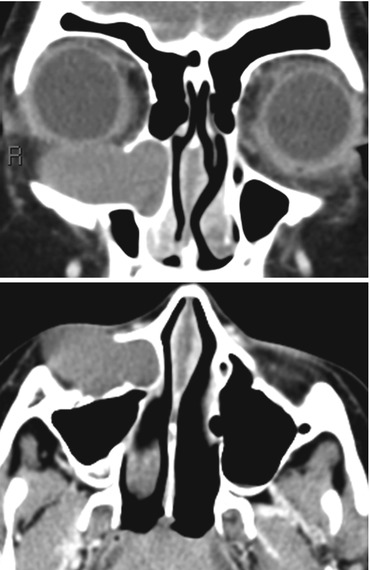

Fig. 13.5
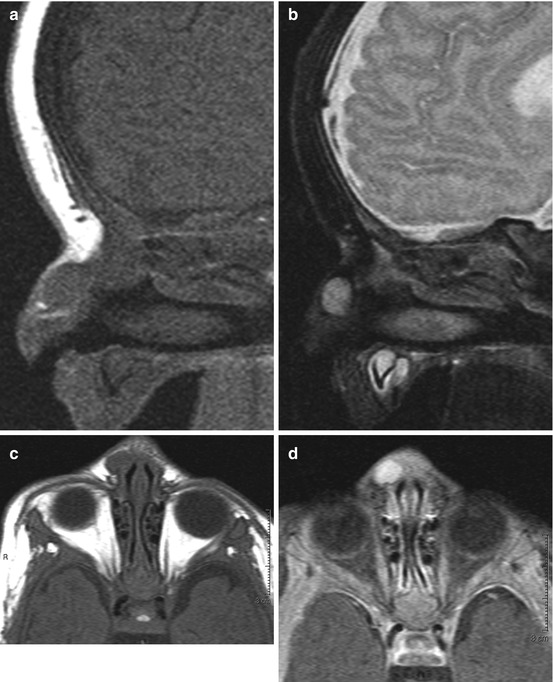
Dacryocystocele. Dacryocystoceles may be unilateral or bilateral, with the latter tending to present at birth with respiratory distress. Unilateral lesions may go undetected until later in childhood. A smoothly expansile lesion, conforming to the course of the nasolacrimal duct is the typical appearance of a dacryocystocele (Images courtesy of C. Douglas Phillips, MD)
13.2.4 Midline Nasal Anomalies
Box 13.2 summarizes the embryological mechanisms that underlie the four types of midline nasal anomalies (Figs. 13.6, 13.7, and 13.8) and their typical imaging features. Each of these may present as a sinus or as a midline mass. An encephalocele is likely when the mass increases in size with crying. A firm mass may be a nasal heterotopia or a dermoid. Nasal glial heterotopias (also called nasal gliomas) are nonneoplastic disorganized rests of brain tissue that present as firm midline nasal masses and are best investigated with a combination of CT and MRI. The most important determination is if there is communication with the cranial cavity. With larger encephaloceles, this is usually not problematic and the herniation of meninges, CSF, and brain tissue through the midline frontonasal defect is usually obvious on MRI. Intracranial extension may not be as obvious in the case of dermal sinuses, nasal gliomas, and dermoid/epidermoid cysts. The presence of a widened foramen cecum or of a bifid crista galli is indicative of intracranial extension.
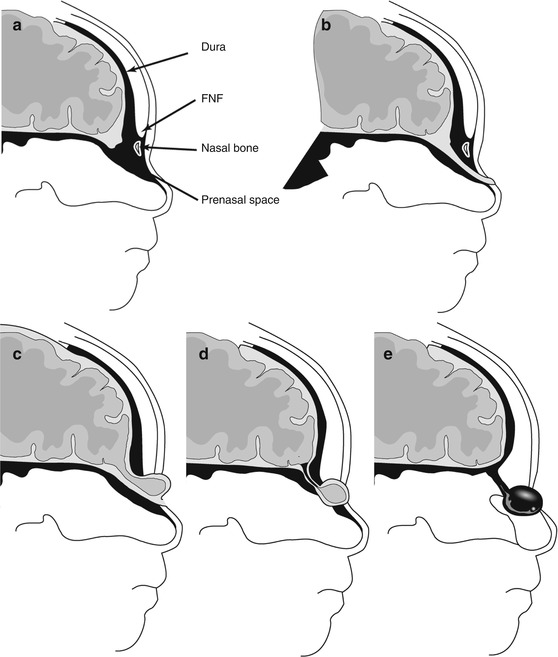
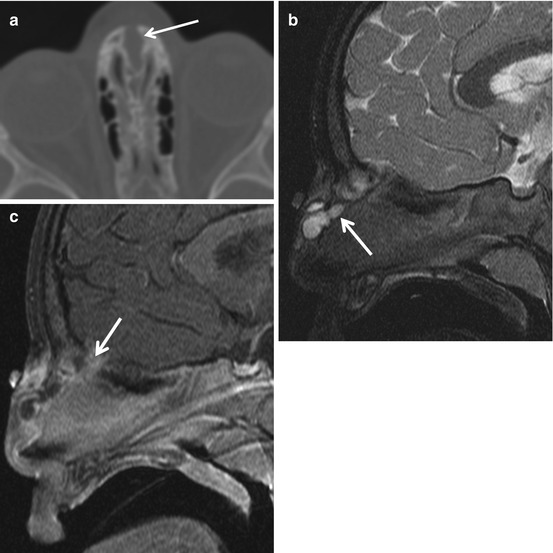

Fig. 13.6
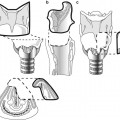
Midline nasal anomalies. (a) Normal development. FNF fonticulus nasofrontalis – a transient opening that closes during normal development. FC foramen cecum. (b) Nasal dermal sinus. (c) Encephalocele. (d) Nasal cerebral heterotopia (nasal glioma). (e) Dermoid cyst. Modified from Lowe et al. (2000)

Fig. 13.7
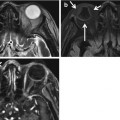
Infected nasal dermal sinus with dermoid. The sinus tract (arrow) at surgery was found to extend between the nasal bones and split the crista galli (a). Also, a lobulated dermoid cyst, as demonstrated on the sagittal T2 (b) and contrast-enhanced T1 (c), was found. In (c), the intracranial extent of the sinus tract is evident. The enhancement along the sinus tract was due to infection. Dermoid and epidermoid cysts can coexist with dermal sinuses (Images courtesy of C. Douglas Phillips, MD)