Pediatric Neuroimaging
Camilla Lindan
Erik Gaensler
Jerome Barakos
Pediatric neuroimaging is one of the most fascinating of specialties, calling upon our knowledge of embryology, genetics, and biochemistry. Neurodevelopmental changes occurring in utero continue through the early postnatal years, resulting in striking alterations to the appearance of the brain on imaging studies from term through 2 years of age. Injuries such as ischemia or infection result in different patterns of imaging as a function of age and inborn errors of metabolism often present in early childhood. This chapter discusses normal development, hypoxic ischemic injury, congenital malformations, and the phakomatoses. Pediatric CNS tumors, white matter diseases, and spine malformations are discussed in separate chapters.
MR has established itself as the procedure of choice for pediatric neuroimaging, although specific situations for which US remains advantageous will be described. CT scanning is primarily employed for evaluation of trauma, the skull, or calcifications. Concerns regarding radiation risk to the young brain limit the use of CT. MR scanners are designed to image adult patients, and special arrangements must be made to accommodate the pediatric population. Sedation is usually required for children younger than 6 years of age, and pediatric sedation protocols should be performed in conjunction with the pediatric medicine service. For imaging of neonates, sedation may not be necessary, but careful coordination between the clinical and imaging staff is essential to ensure patient safety and optimal imaging. Imaging of ill neonates requires MR-compatible support systems for providing heat, oxygen, IV drugs, and monitoring.
Normal Patterns of Sulcation and Myelination
Any discussion of pediatric neuroimaging should begin with normal development and myelination as a frame of reference. Before interpreting any exam on a young child, it is important first to know the corrected gestational age because it is only against this background that we may accurately interpret neuropathology. The advent of fetal MR and increasingly frequent imaging of premature infants necessitate familiarity with the changing appearance of the brain from the second trimester through 2 years of postnatal life.
Premature Infant. The most striking changes observed on imaging from 18 weeks’ gestational age through term relate to cortical infolding. At 24 weeks, the brain is essentially smooth with mild indentation of the sylvian and parietooccipital fissures only. By 38 weeks, an adult gyral pattern is established (Fig. 8.1). Although myelination is progressing during this time, the T1 and T2 imaging appearances of gray and white matter are relatively stable. Head ultrasound (HUS) is the most common modality used to image premature infants (Fig. 8.2). As will be seen in a later discussion of myelination and migrational abnormalities, it is important to first note the corrected gestational age of the infant before interpreting pediatric neuroimaging, as failure to do so may result in errors of diagnosis.
Term Infant. At birth in the normal term neonate, the relative signal intensities of the white and gray matter are the inverse of the “adult pattern.” This is because the amount of free water, the source of mobile hydrogen protons that form the basis of the MR signal, is high. The myelin sheath (a lipid) is hydrophobic. As axons myelinate, free water decreases and over the first 2 years of life a well-established pattern of progressive T1 and T2 shortening is observed.
Myelination signal changes occur earlier on T1WI than on T2WI. T1WI best provides a detailed view of actively myelinating structures in the first year. Areas that become myelinated stand out as high signal on T1WI against a background of low-signal-intensity unmyelinated white matter. By 8 months, the T1WI demonstrate essentially an adult pattern though the T2WI have changed minimally. Note that myelination on T2WI will not approach the adult pattern for another 10 months, that is, adult pattern on T2WI by approximately 18 months. From 8 through 18 months, the T2WI are most useful to follow myelination patterns. Heavily weighted T2 images (TR 3000, TE 60/120) are recommended for the age range of 0 to 12 months. As the water content of the infant brain is high, heavily T2WIs are needed to discriminate between many brain structures that have similarly long T2 relaxation times. With growth, the white matter will assume the adult low signal intensity pattern on T2WIs by 18 months, with some high signal intensity persisting in the terminal myelination zones on T2WIs.
Familiarity with the normal patterns of myelination at term is especially important, as will become evident during the later discussion of hypoxic–ischemic injury (HII). At term, T1
and T2 shortening is observed in myelinated areas such as the corticospinal tracts, dorsal brain stem, and the ventrolateral thalamic nuclei. It is particularly important to note the normal small foci of low signal myelinated white matter within the PLIC on T2WI at term. Subtle T2 shortening is also observed in the rolandic cortex and dorsal brain stem (Fig. 8.3).
and T2 shortening is observed in myelinated areas such as the corticospinal tracts, dorsal brain stem, and the ventrolateral thalamic nuclei. It is particularly important to note the normal small foci of low signal myelinated white matter within the PLIC on T2WI at term. Subtle T2 shortening is also observed in the rolandic cortex and dorsal brain stem (Fig. 8.3).
Over the first 18 months of life, in general, myelination proceeds from dorsal to ventral, from caudad to cephalad, and from central to peripheral. The anterior limb of the internal capsule should develop high signal intensity on T1WIs by 3 months, the splenium of the corpus callosum becoming bright on T1WIs by 4 months, and the genu by 6 months. As the peripheral white matter gradually transitions in appearance to adult signal, it will go through stages (on both T1 and T2) during which it is isointense to gray matter. At such times, evaluation for anomalies such as cortical malformations is limited (Fig. 8.4). T2 signal changes are more subtle during the first year, observed in the deep and central white matter and corpus callosum.
An important time point to remember is 8 months, by which time the brain should essentially have a normal adult appearance on T1WI (Fig. 8.5). T2 signal changes are more subtle during the first year, observed in the deep and central white matter, and corpus callosum. The unmyelinated peripheral white matter remains high signal on T2WI during the fist year.
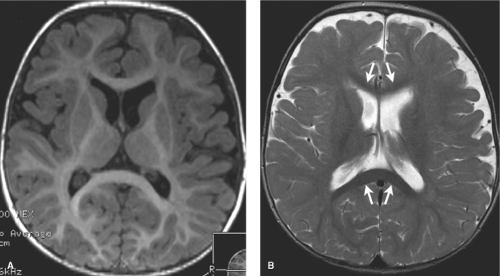
A second important time point to remember is 18 months. At this age, the white matter has essentially assumed the adult low signal intensity on T2-weighted images (Fig. 8.6). Mild symmetric high signal intensity persists on T2WI beyond 18 months in the terminal myelination zones adjacent to the atria of the lateral ventricles on T2WIs. It is important not to confuse these with areas of brain injury as is discussed in the section on neonatal encephalopathy (NE).
A CT of a neonatal brain will appear quite different from that of a young child. Low attenuation (watery) normal white matter should not be interpreted as edematous or indicative of a leukodystrophy (Fig. 8.7).
One way to help remember the stages of myelination is that myelination parallels developmental landmarks. Newborns can breathe and have function of the motor components of the cranial nerves—all medullary and pontine tracts. Motor functions that allow the child to roll over, crawl, and stand develop in the first year, paralleling the myelination of the internal capsule. Higher cortical functions such as speech are the last to appear, tracking with the maturation of the hemispheric white matter in the second year of life. It is helpful to have at hand a reference of normal appearances for age against which any scan from birth through 18 months is compared. Several excellent references are provided at the end of the chapter. To
reiterate, evaluation of every pediatric brain MR image must begin with establishment of the corrected gestational age and an assessment of sulcation and myelin development.
reiterate, evaluation of every pediatric brain MR image must begin with establishment of the corrected gestational age and an assessment of sulcation and myelin development.
Neonatal Encephalopathy
Neonatal encephalopathy (NE) refers to a clinically defined syndrome of neurological dysfunction in the term and near-term infant. A wide variety of etiologies may account for this state including metabolic conditions, maternal and fetal infections, drug exposure, hypoxic ischemic injury, neonatal stroke, congenital CNS malformations, and other disorders. Thus, the general, all-encompassing term of neonatal encephalopathy is often favored to the more specific term of Hypoxic Ischemic Injury (HII), which refers to the clinical syndrome relating specifically to brain damage mediated by hypoxia or ischemia. It cannot be overemphasized that HII is but a subset of neonatal encephalopathy, which has a long list of possible causes. Many cases of NE have an associated antepartum in utero or metabolic foundation.
Hypoxic Ischemic Injury
Though the incidence is decreasing, hypoxic ischemic injury (HII) is still not uncommon in the pediatric population, with each pediatric center seeing multiple cases annually. Causes are multiple and include placental pathology, infection and metabolic disorders as well as more obvious etiologies such as placental abruption. The end product of these varying disorders may be some degree of hypoxia and ischemia which ultimately injures the brain. Early imaging findings of HII can be very subtle and familiarity with the specific patterns is essential so that one may focus attention to the areas of the brain most commonly injured.
Observed patterns of injury vary depending on a multitude of factors, a complete discussion of which is beyond the scope of this text. These include the following:
Severity of injury
Duration of injury.
Gestational Age: The maturity of the brain influences the observed patterns of injury for many reasons including the following
Ability of vessels to autoregulate intracerebral blood flow
Selective vulnerability of different areas of the brain: Based on metabolic differences such as function and concentration of neurotransmitters, receptors, glucose metabolism, myelination
Secondary energy failure and delayed apoptosis
Damage to other organ systems which can result in secondary effects such as dimished cardiac output
Time at which scans are acquired relative to the time of injury
Treatment with neuroprotective agents.
Before evaluating any scan, first establish the corrected gestational age of the infant. Recall how developing sulcation and myelination patterns dramatically change the appearance of the premature and neonatal brain. One must be familiar with these evolving normal background appearances of the brain at various stages of development otherwise subtle abnormalities may be missed or misinterpreted. Importantly, as we discuss in detail later, the imaging appearance will be highly dependant on the time at which the study is acquired relative to the time of injury, whether evaluated in the acute, subacute, or chronic phase. Though initially this may seem a complicated topic indeed, if one is familiar with various patterns, a careful search approach on imaging will insure that significant abnormalities are not missed. When reading the following discussion, remember that overlap can occur between these differing patterns which represent a continuum from prematurity through term.
Hypoxic Ischemic Injury in the Premature Neonate. Premature infants, defined as less than 36 weeks’ gestational age, have not yet developed the autoregulatory capacity needed to protect their brains from the blood pressure and perfusion fluctuations they experience in the ex utero environment. This situation is termed a “passive flow state,” where variations in systemic pressure, whether high or low are directly transmitted to the CNS circulation. As such, ischemia is exacerbated by conditions that affect systemic pressure in the premature infant such as respiratory distress syndrome, pneumothorax, patent ductus arteriosus, and sepsis.
Most often observed are the effects of mild to moderate hypoxia on the premature brain. These include germinal matrix (GM) and intraventricular hemorrhages (IVH) as well as injury to the periventricular white matter. Incidence is inversely related to birth weight, with 25% of infants less than 2,000 g experiencing intraventricular hemorrhage in the early postnatal period.
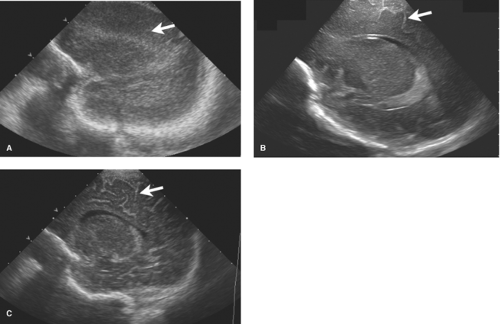
HUS is the preferred modality to image the brains of premature infants. Its portability is ideal for evaluation of infants in the NICU for whom a controlled environment must be maintained. The open fontanelles allow excellent evaluation of the ventricles and central brain. Standard images are acquired through the anterior fontanelle with supplemental images obtained through the posterior and mastoid fontanelles. HUS diagnosis of HII is described in detail in Chapter 38 and only a brief review is provided here.
The germinal matrix is the source of the majority of hemorrhages in premature infants. During neurodevelopment, the germinal matrix, which lines the ventricles, gives rise to neurons that migrate outward to the cortex. It is composed of highly vascular tissue, containing large premature capillaries lined by only simple endothelium. Metabolically active during development, the germinal matrix is highly susceptible to hypoxia and when injured, its capillary integrity is easily disrupted.
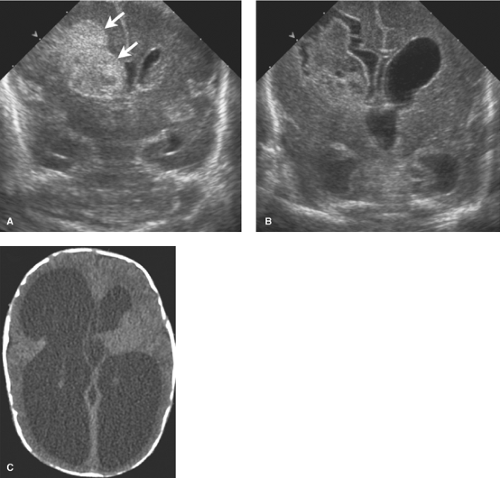
Germinal matrix and intraventricular hemorrhages are classically divided into four grades (see Table 38.4 and Figs. 38.25 to 38.28). By 34 weeks, the GM has largely involuted with residual tissue seen at the inferior aspect of the lateral ventricular bodies in an area known as the caudothalamic groove or notch. The mildest hemorrhages are confined to the GM in the notch, termed “Grade I.”
Grade II bleeds are defined as those that extend into the ventricles but do not distend them. When the ventricles enlarge due to either increased bleeding or secondary obstructive hydrocephalus, the hemorrhage is described as Grade III. Grade IV bleeds, initially thought to be a result of extension of IVH into the surrounding brain, are now known to represent hemorrhagic venous infarctions in the periventricular white matter. The unmyelinated premature brain is delicate and when under the pressure of hydrocephalus the periventricular tissues may atrophy rapidly resulting in further ventricular expansion (Fig. 8.8). Serial HUS are used to follow premature infants with GM and IVH. MR provides the most detailed evaluation of HII but monitoring ill premature infants for lengthy MR exams is sometimes difficult. CT is less preferable due to radiation exposure.
Periventricular white matter injury of prematurity is also sometimes still referred to by the term “periventricular leukomalacia” or PVL. Selective vulnerability of periventricular preoligodendrocytes to injury, mediated by glutamate, has been established to primarily account for the patterns of damage observed. The relative hypovascularity of the periventricular zones in the second and early third trimesters likely also plays a role though less than previously thought. PVL is common in early premature infants but difficult to diagnose on HUS. Initially, subtle zones of increased echogenicity are observed, typically in the white matter adjacent to the atria of the lateral ventricles. This may resolve on HUS or progress to frank small areas of cavitation due to cystic encephalomalacia. On MR, diffusion-weighted imaging (DWI) shows early restriction in the white matter, which resolves within 5 to 7 days (Fig. 8.9). At 2 to 5 days after injury, multiple small nonhemorrhagic foci of high signal on both T1WI and T2WI may be observed
within the periventricular white matter. After 1 week, these evolve and may appear dark on T2WI.
within the periventricular white matter. After 1 week, these evolve and may appear dark on T2WI.
The mature brain responds to injury with areas of scarring, termed “gliosis,” which will appear high in signal on T2WI. The capacity to develop gliosis does not develop until 28 to 30 weeks and destructive lesions in utero or young premature infants may result in areas of cavitation or porencephaly with little adjacent high T2 signal scar.
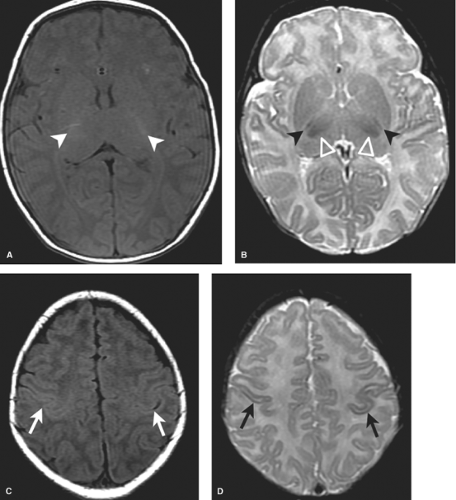
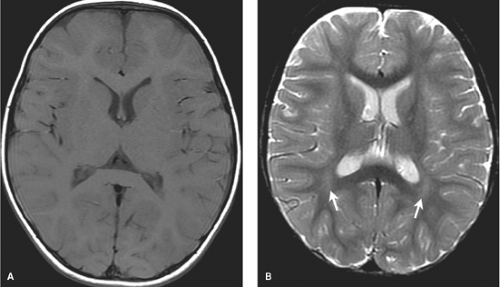
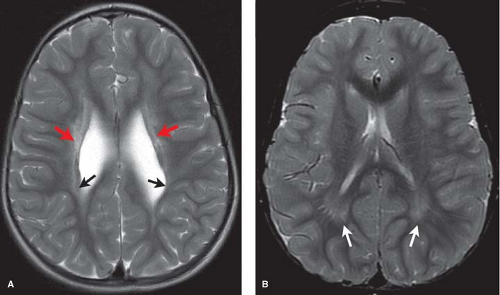
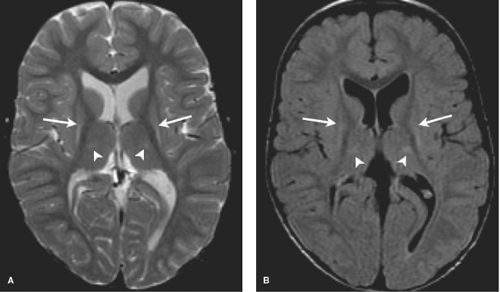
MR most accurately diagnoses PVL in the chronic stages when areas of periventricular white matter volume loss with or without gliosis are observed. Focal areas of chronic white matter gliosis are most accurately discovered after 18 months corrected gestational postnatal age when they will stand out on T2WI and fluid-attenuated inversion recovery (FLAIR) as areas of high signal against the background of normal low signal “adult pattern” white matter tracts (Fig. 8.10).
When located in the periatrial white matter, chronic residua of mild PVL may be difficult to distinguish from the “terminal zones” of unmyelinated white matter observed in normal children well beyond 18 months of age. Helpful clues to accurately diagnose gliosis because of prior PVL are (1) thin white matter between the dilated lateral ventricles and cortex indicating atrophy; (2) increased conspicuity of gliosis relative to unmyelinated white matter on proton density images; (3) gliosis may immediately contact the ventricular margin whereas terminal zones are separated by a thin zone of normally myelinated white matter (Fig. 8.11).
Again, it should be emphasized that the finding of imaging abnormalities compatable with PVL may be the result of many different conditions, including infection. In particular, in the last decade, intrauterine infection and inflammation have been identified among the causes of preterm delivery and its complications. Maternal, placental, or amniotic infections may result in the production of cytokines, which gain access to the fetal circulation and result in a systemic fetal response termed “FIRS” (fetal inflammatory response syndrome). This condition has been implicated as a cause of fetal and neonatal injury that leads to PVL and when severe, the clinical syndrome of cerebral palsy.
When hypoxic injury to the premature infant is profound, the brain stem and deep gray tissues, especially the thalami, are also damaged. Such injuries are most accurately diagnosed by MR (Fig. 8.12).
Hypoxic ischemic injury in the term infant (36 weeks’ gestation and older) results in distinct patterns on MR. It must be
remembered that HII has many etiologies including infections, placental pathology as well as varied inflammatory and metabolic processes. The radiologist needs to be familiar with various observed imaging patterns to focus attention to those areas of the brain most commonly injured as even profound damage to the brain can have very subtle appearances in its early stages. Injuries are also often symmetric, which makes them more difficult for us to perceive. Complicating matters further, observed patterns of HII evolve quickly over time during the first weeks after injury. When imaged days apart, the same injury may have vastly different appearances. The MR sequences upon which we most rely on to diagnose HII in adults do not always work in familiar ways in neonates. Interventions such as hypothermia or other neuroprotective strategies can alter the outcomes and observed MR findings. In order to accurately diagnose neonatal HII, familiarity with all of these complex factors is essential.
remembered that HII has many etiologies including infections, placental pathology as well as varied inflammatory and metabolic processes. The radiologist needs to be familiar with various observed imaging patterns to focus attention to those areas of the brain most commonly injured as even profound damage to the brain can have very subtle appearances in its early stages. Injuries are also often symmetric, which makes them more difficult for us to perceive. Complicating matters further, observed patterns of HII evolve quickly over time during the first weeks after injury. When imaged days apart, the same injury may have vastly different appearances. The MR sequences upon which we most rely on to diagnose HII in adults do not always work in familiar ways in neonates. Interventions such as hypothermia or other neuroprotective strategies can alter the outcomes and observed MR findings. In order to accurately diagnose neonatal HII, familiarity with all of these complex factors is essential.
Patterns of HII have traditionally been distinguished as profound acute (PA) HII or prolonged partial (PP) HII. In practice, ischemic injury is a continuum and overlap often occurs. Nonetheless, this distinction provides a useful framework for learning the various patterns of HII observed in the term neonate.
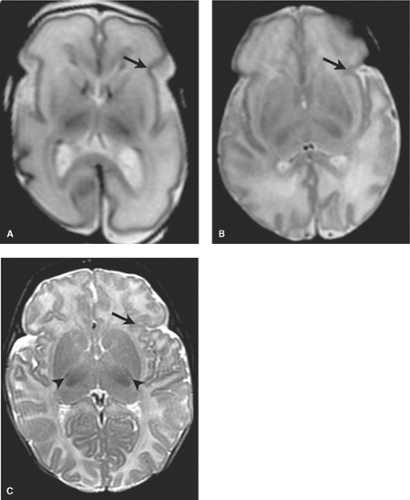
Profound Acute (PA) Perinatal HII at Term: Basal Ganglia Thalamic Pattern of Injury (BGT). Profound HII usually occurs in the setting of a sentinel event such as placental abruption or uterine rupture, which may cause acute near total asphyxia. The selective vulnerability of metabolically active areas results in damage to vital central brain areas with relative sparing of the majority of the cerebral cortex. On MR imaging, one observes what is termed “the basal ganglia thalamic pattern” (BGT.) Typically, the ventrolateral thalami, posterior putamina, the globi pallidi, and the intervening posterior limbs of the internal capsule are injured. Additional involvement is often observed within the corticospinal tracts extending to the sensorimotor cortices. Other metabolically active areas such as the hippocampi, lateral geniculate nuclei,
and dorsal brain stem may also be injured. Recall that these are the areas actively myelinating as observed on term neonatal MR (Fig. 8.1).
and dorsal brain stem may also be injured. Recall that these are the areas actively myelinating as observed on term neonatal MR (Fig. 8.1).
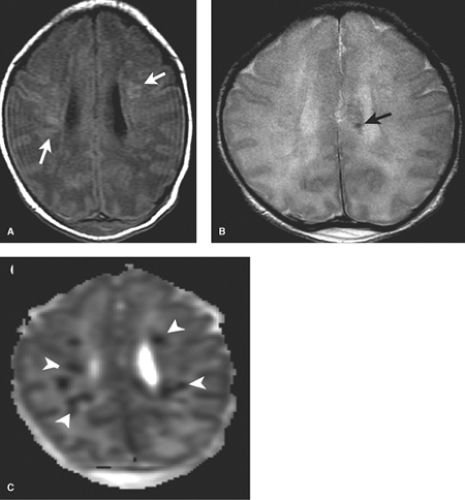
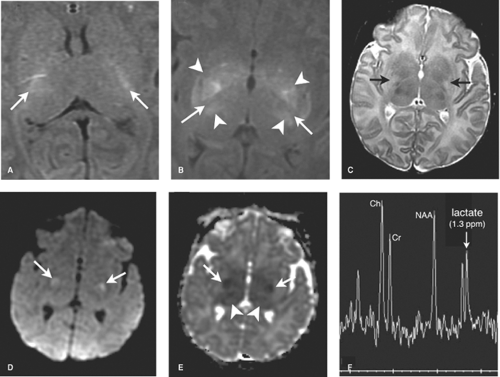
DWI is initially the most sensitive series, abnormal within the first 24 hours. Diffusion restriction, demonstrated primarily in the BGT, and corticospinal tracts extending to the perirolandic cortex, is often more conspicuous on the apparent diffusion coefficient (ADC) map given the high water content of the white matter in infancy (Fig. 8.13). DWI may, however, initially underestimate the extent of injury (likely due to apoptosis) and normal early DWI has been reported. DWI restriction peaks at 3 to 5 days and “pseudonormalizes” by the end of the first week, a term used as it does not indicate reversal of injury. Apoptosis may also account for fluctuating patterns: areas of new restriction and simultaneous pseudonormalization may be observed during the first week.
MR spectroscopy can be a useful and sensitive tool, demonstrating elevated lactate, which may be the only abnormal finding in HII during the first 24 hours. Use of long TE technique (TE = 288 msec) maximizes sensitivity to lactate. Decreased NAA and elevated a-glutamate/glutamine peaks portend a worse prognosis. It must be noted that it is normal to observe a lactate peak before 37 weeks’ gestational age, and in term neonates, a small lactate peak may be seen. In general, following an ischemic insult, lactate increases to a maximum at 5 to 6 days and then diminishes. Persistently elevated lactate levels greater than a month following birth have been reported following perinatal hypoxia–ischemia and postulated to be the result of persistent abnormal metabolism in the injured regions of brain.
T1WI and T2WI in the first 12 hours are typically unremarkable. After 12 to 24 hours, they begin to become abnormal though often in subtle ways. In more severe insults, the time of onset for detection of injury can be shifted several
hours earlier. Detection of these early findings requires familiarity with the normal appearance of the term neonatal brain. Recall that myelinated tissues such as the posterior limbs of the internal capsules (PLIC) the ventrolateral nuclei of the thalami (VLNT), the posterior lentiform nuclei, the corticospinal tracts, and perirolandic cortex are intrinsically bright on term T1W neonatal scans. T2WI demonstrate corresponding similar subtle foci of low signal in the normal term neonate, of which one of the most important to check for is in the PLIC.
hours earlier. Detection of these early findings requires familiarity with the normal appearance of the term neonatal brain. Recall that myelinated tissues such as the posterior limbs of the internal capsules (PLIC) the ventrolateral nuclei of the thalami (VLNT), the posterior lentiform nuclei, the corticospinal tracts, and perirolandic cortex are intrinsically bright on term T1W neonatal scans. T2WI demonstrate corresponding similar subtle foci of low signal in the normal term neonate, of which one of the most important to check for is in the PLIC.
In the first 1 to 3 days following HII, profound ischemia may result in a reversal of the normal T1 and T2 signal in the PLIC (Fig. 8.13). The VLNT and the posterior lentiform nucleus are also preferentially damaged and will demonstrate mottled mixed increased T1 and T2 signal (Fig. 8.6). Since these nuclei are located just adjacent to the PLIC, they can make identification of PLIC signal abnormality difficult, and close inspection is required. Loss of normal T1 and T2 signal in the PLIC has been determined to be predictive of adverse outcomes.
Similar subtle abnormal signal to that observed in the thalami and basal ganglia may be present in the tegmentum of the midbrain, dorsal brain stem, lateral geniculate nuclei, cerebellum, and hippocampi. After the second week, deep gray structures often develop T2 shortening.
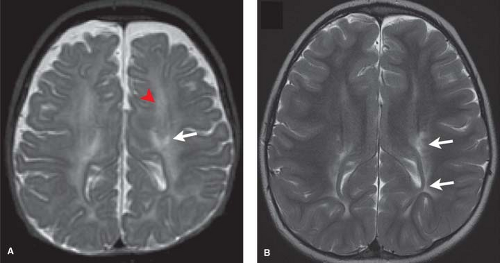
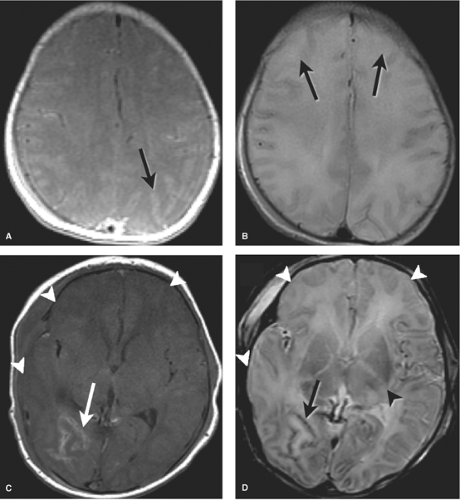
The corticospinal tracts and perirolandic cortex, highly metabolically active at term, are often affected. Initially normal, within 2 days T1- and T2-weighted image abnormalities are first evident, becoming more conspicuous over the next several days. The appearance in the injured areas of cortex is often that of an accentuation of the normally present T1 shortening, which can give the cortex a “highlighted” appearance, persisting for up to several months. On the corresponding T2WI, careful inspection of the gray–white junction of the affected cortex will at first demonstrate blurring due to edema (T2 prolongation). This is more difficult to perceive in infants due to the intrinsic high T2 signal of adjacent unmyelinated white matter. Careful inspection of T2WI may also reveal the white matter within the corticospinal tracts, standing out as especially bright, more so than the unaffected white matter or even the CSF within the ventricle. After 7 days, a shift may occur and T2 shortening, or low signal, may predominate within affected cortex (Fig. 8.14).
The development of high T1 signal in areas of acutely injured brain, seen in both the cortex and deep structures, reflects an important distinction from the pattern of cytotoxic edema observed in older children and adults. The reasons for this increased T1 signal are not fully understood but may reflect the presence of microhemorrhage, myelin breakdown with lipid release, mineralization, or free radicals. These areas of abnormal T1 shortening may be difficult to perceive against the background of the intrinsic high T1 signal expected due to normal myelination.
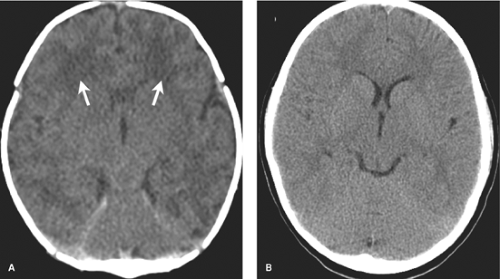
FLAIR images, so useful in older children and adults, are less so in infants due to intrinsic high signal of unmyelinated brain, which masks the appearance of adjacent cytotoxic edema. MR is the most sensitive and preferential modality for imaging in the setting of HII but if CT is acquired, it may show subtle symmetric loss in density of the basal ganglia and thalami.
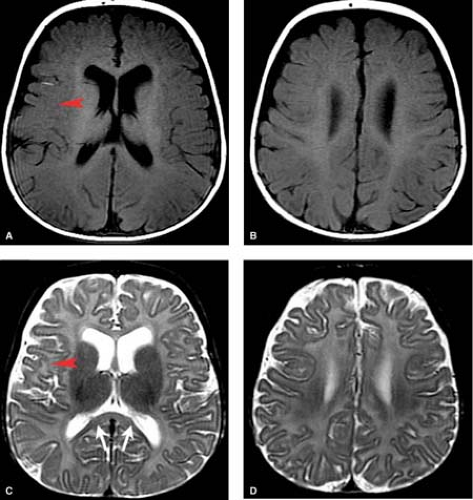
In the subacute phase, after 1 to 2 weeks, DWI are insensitive and the T1 and T2 images are most useful. At this stage, both T1 and T2 shortening is often observed in injured tissues. In the chronic phase injured tissues will demonstrate atrophy and gliosis (high signal on T2WI) (Fig. 8.15). Children with basal ganglia thalamic pattern of injury tend to be severely disabled with dyskinetic cerebral palsy.
If a child sustains ischemic injury after 4 months of age, profound hypoxia tends to injure all of the basal ganglia and a much larger proportion of cerebral cortex with relative sparing of the thalamus and perirolandic regions.
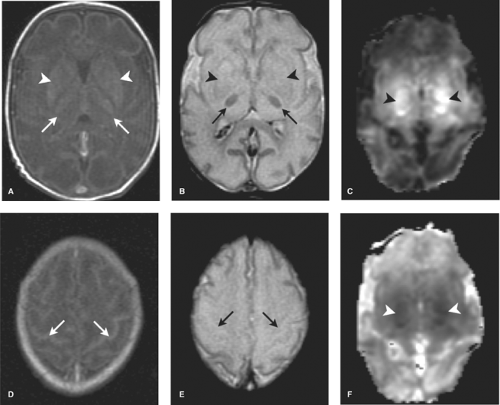
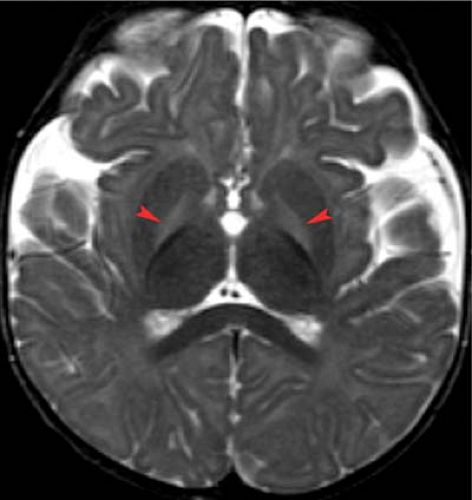
It is important to remember that not all imaging abnormalities observed in the basal ganglia or thalami can be ascribed to HII. These regions are also extremely sensitive to toxic and metabolic processes which in the appropriate clinical settings need to be considered in the differential diagnosis (Fig. 8.16). (Also, please see metabolic and toxic section in Chapter 7.)
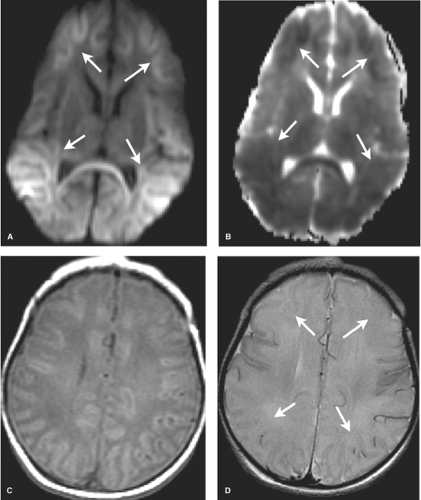
Partial Perinatal HII: Watershed Pattern of Injury (WS). Partial or milder hypoxic ischemic events in the perinatal period, such as a nuchal cord, tend to spare central brain areas and will damage more peripheral gray and white matter. This is because the “diving reflex” redistributes blood to the most metabolically active areas such as the corticospinal tracts, basal ganglia, thalamus, and brain stem. These peripheral infarcts are typically located in the interarterial parasagittal border zones between the anterior, middle, and posterior cerebral arteries, termed “watershed.”
In the acute setting, findings evolve over the first days. Features are similar to those seen in perirolandic cortical injury in the setting of profound ischemia discussed earlier. Images in the first 24 hours may appear normal with only the MRS detecting elevated lactate. Diffusion images are sensitive in the first 24 hours but may underestimate the extent of injury as in profound HII. Careful inspection of the gray white junction may demonstrate blurring due to T1 and T2 prolongation, more difficult to perceive in infants due to the intrinsic low T1 and high T2 signal of the adjacent unmyelinated white matter. However, alternate patterns may be observed with accentuation of T1 and T2 shortening, making the cortex appear highlighted (Fig. 8.17).
Chronic findings such as atrophy and gliosis are observed in watershed zones of the peripheral brain. As the depths of the gyri are preferentially affected, the appearance of the shrunken gyri has been likened to that of mushrooms, a term called “ulegyria” (Fig. 8.18).
Neuroprotective Strategies and Imaging. Therapeutic hypothermia has proven to be an effect tool in the treatment of neonates with HII, with studies confirming improvement in end-point measures at 18 to 22 months. Neonates who undergo cooling reveal milder imaging features of HII with a decreased incidence of deep gray matter and cortical lesions. Trials are ongoing as neurological outcomes at 18 to 22 months may not reflect the true long-term benefits (Fig. 8.19).
Imaging Pearls: HII in the Term Infant. Acute hypoxic–ischemic damage can be difficult to discern in newborn infants. Some helpful hints follow:
Be familiar with the normal pattern of myelination in term neonates and look carefully for subtle edema in the PLIC in the setting of profound HII. Remember that bilateral symmetric findings, especially when subtle, are often the most difficult for us to perceive.
T1WIs can be a source of confusion. Do not confuse normal active myelination (basal ganglia, thalami, cerebral peduncles, perirolandic white matter) with the abnormal T1 shortening observed in profound HII.
Unmyelinated white matter is bright on T2WIs, making infarcts less apparent—like looking for watery areas in an ocean. Careful inspection of the cortical ribbon is required
to identify subtle areas of blurring due to edema, called the “missing cortex sign.” Also look for subtle areas of extremely bright white matter along the CST.
FLAIR sequences are not helpful, and DWI is most sensitive early. MR spectroscopy has become a useful tool, as lactate elevation may be the only abnormal finding, particularly early on. After 1 week when diffusion images pseudonormalize and lactate peaks resolve, T1WI and T2WI are most useful.
Though distinction between profound acute and prolonged partial HII provides a useful framework, in practice overlap occurs and when injury is very severe a combination of both patterns is observed, resulting in a DWI “superscan.”
Obtain a good clinical history: gestational age, birth history, onset and type of symptoms, time which has elapsed from the suspected injury to the scan and whether any neuroprotective strategies were employed. Remember that not all NE is due to HII and metabolic, congenital,
and infectious etiologies may need to be considered, especially when imaging findings or clinical symptoms are atypical.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree