Pelvicalyceal System, Ureters, Bladder, and Urethra
William E. Brant
Pelvicalyceal System and Ureter
Imaging Methods
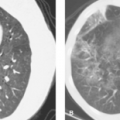
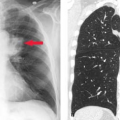
As described in Chapter 32 the CT urogram (“CT IVP”) is now the imaging method of choice for evaluation of hematuria and a screening examination of the pelvicalyceal system and ureters (1,2,3,4,5). Thin slice MDCT acquisitions are reformatted in longitudinal planes to provide visualization of the collecting system comparable to the traditional IV pyelogram (IVP), also called the excretory urogram. The CT urogram is limited by lower spatial resolution than the IVP, which is based on traditional radiography (see Fig. 32.17). The CT urogram is also limited when contrast opacification of the collecting system and the ureters is incomplete. However, the improved contrast resolution and demonstration of soft tissues makes CT urogram a high-quality diagnostic study despite its limitations. The MR urogram may be substituted for the CT urogram. The MR urogram may be performed with gadolinium administration producing a full evaluation similar to the CT urogram (1,6). However, whenever contrast administration is contraindicated, an MR urogram can be performed without contrast by utilizing heavily T2WI. The high signal from urine in the collecting systems, ureters, and bladder closely resembles a contrast-enhanced study (see Fig. 33.8).
Retrograde pyelography, performed by cystoscopic catheterization of the ureteral orifice followed by injection of contrast, is independent of renal function, provides high-quality images of the ureter and the collecting system, and is another alternative commonly utilized by urologists. When a percutaneous nephrostomy catheter has been placed in the collecting system, antegrade pyelography is an additional choice. US is the imaging method of choice for screening for hydronephrosis but is limited in its ability to demonstrate small uroepithelial tumors. MDCT, utilizing thin slices and performed without IV or oral contrast agents, has replaced plain radiographs and the traditional IVP for the diagnosis of renal stones in the kidneys and the ureters.
Anatomy
The collecting tubules of a medullary pyramid coalesce into a variable number of papillary ducts that pierce the tip of the papilla and drain into the receptacle of the collecting system called a minor calyx. The projection of a papilla into the calyx produces a cup shape. The sharp-edged portion of the minor calyx projecting around the sides of a papilla is called the fornix of the calyx. Compound calyces, usually found at the poles of the kidney, are formed by the projection of two or more papilla into the calyx. Infundibula extend between minor calyces and the renal pelvis. The renal pelvis is triangular, with its base within the renal sinus. The apex of the pelvis extends outward and downward to join the ureter. A so-called extrarenal pelvis is predominantly outside the renal sinus and is larger and more distensible than the more common intrarenal pelvis, which is surrounded by renal sinus fat and other structures (Fig. 33.1). An extrarenal pelvis is a normal variant that should not be confused with hydronephrosis. There is endless variety in the size and arrangement of calyces and in the shape and appearance of the renal pelvis.
The ureters have an outer fibrous adventitia that is continuous with the renal capsule and the adventitia of the bladder. The muscularis, responsible for ureteral peristalsis, consists of outer circular and inner longitudinal muscle bundles. The mucosa lining the entire pelvicalyceal system, ureters, and bladder is transitional epithelium. The ureters enter the bladder at an oblique angle. When the bladder wall contracts, the ureteral orifices are closed. The ureters propel urine by active
peristalsis, which can be visualized fluoroscopically and by US. Jets of urine opacified by contrast are frequently seen within the bladder on CT. Because of peristalsis, the diameter of the ureter at any particular instant is highly variable. Three main points of ureteral narrowing, where calculi are likely to become impacted, are (1) the ureteropelvic junction (UPJ), (2) the site at which the ureter crosses the pelvic brim, and (3) the ureterovesical junction (UVJ).
peristalsis, which can be visualized fluoroscopically and by US. Jets of urine opacified by contrast are frequently seen within the bladder on CT. Because of peristalsis, the diameter of the ureter at any particular instant is highly variable. Three main points of ureteral narrowing, where calculi are likely to become impacted, are (1) the ureteropelvic junction (UPJ), (2) the site at which the ureter crosses the pelvic brim, and (3) the ureterovesical junction (UVJ).
Congenital Anomalies
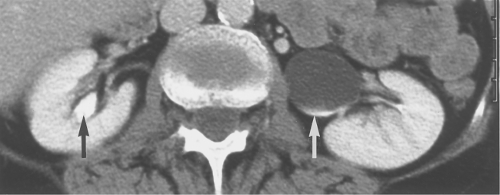
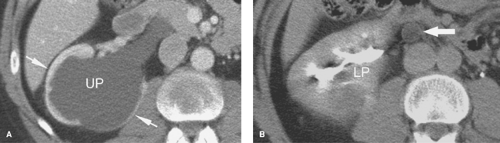
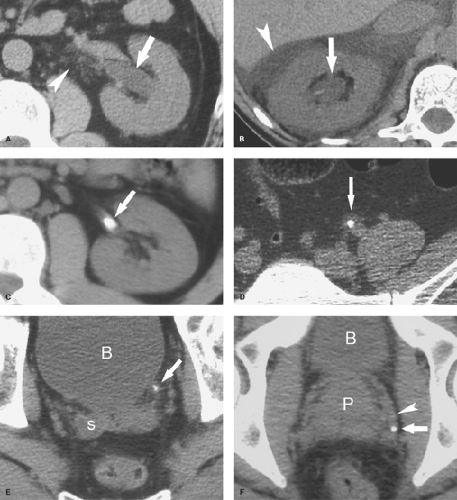
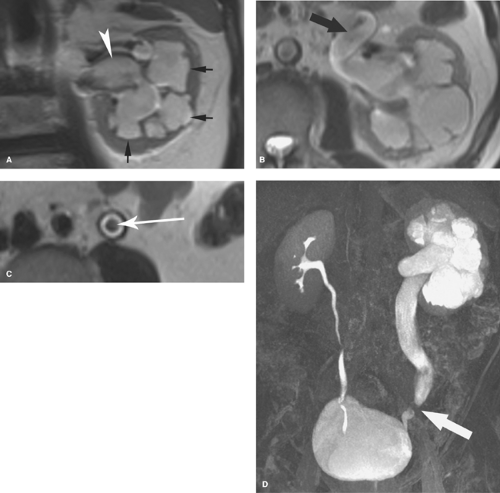
Ureteral duplication occurs in 1% to 2% of the population (Fig. 33.2). Unilateral duplication is six times more common than bilateral duplication (7). The Weigert–Meyer rule states that with complete ureteral duplication, the ureter draining the upper pole passes through the bladder wall to insert inferior and medial to the normally placed ureter draining the lower pole. In females, the ectopic ureter may insert into the lower bladder, upper vagina, or urethra (8). In males, it may insert into the lower bladder, prostatic urethra, seminal vesicles, vas deferens, or ejaculatory duct. The upper pole ureter often ends as an ectopic ureterocele reflecting obstruction because of its ectopic insertion. The lower pole ureter inserts in, or near, the normal location in the bladder trigone and is subject to vesicoureteral reflux because of distortion of its passage through the bladder wall by the ectopic ureterocele (“upper pole obstructs; lower pole refluxes”). Complications of complete duplication include urinary tract infection, vesicoureteral reflux, and UPJ obstruction of the lower pole system. Reflux into the lower pole collecting system in childhood may produce scarring and deformity of the lower pole of the kidney.
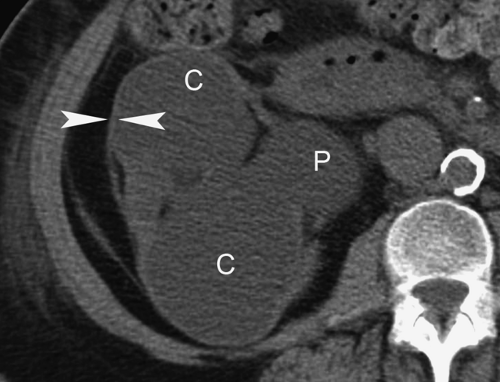
CT or MR urography commonly demonstrate poor function or nonfunction of the obstructed upper pole system (Fig. 33.3). The lower pole system is displaced inferiorly and commonly shows a “drooping lily” appearance. Reflux nephropathy of the lower pole system may be evident. Cystic dilatation of the upper pole system is usually associated with marked parenchymal thinning. The upper pole ureter is commonly tortuous and dilated. The ectopic ureterocele and its associated dilated ureter may simulate a multiseptated cystic mass in the pelvis.
Bifid renal pelvis occurs in 10% of the population. Separate pelvises draining the upper and lower poles join at the UPJ. This anomaly has no pathologic consequences.
Ureteropelvic junction obstruction is a common congenital anomaly that may go undiagnosed until adulthood (9). The amount of hydronephrosis and parenchymal atrophy present
depends on the severity of obstruction. The condition is bilateral in 30% of cases but is often not symmetrical. US demonstrate pelvicalyectasis with sharply defined narrowing at the UPJ. The ureter is not dilated. In 15% to 20% of cases, an aberrant renal vessel causes the obstruction. MDCT is effective in demonstrating the crossing vessel. In the majority of cases, the precise cause is unknown.
depends on the severity of obstruction. The condition is bilateral in 30% of cases but is often not symmetrical. US demonstrate pelvicalyectasis with sharply defined narrowing at the UPJ. The ureter is not dilated. In 15% to 20% of cases, an aberrant renal vessel causes the obstruction. MDCT is effective in demonstrating the crossing vessel. In the majority of cases, the precise cause is unknown.
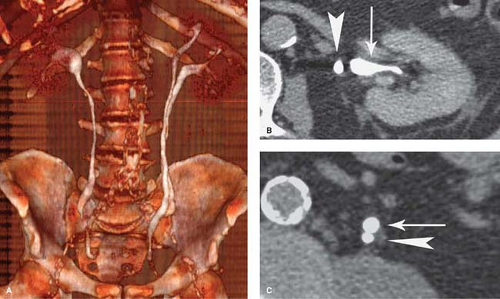
Retrocaval ureter is a developmental variant in which the right ureter passes behind the inferior vena cava at the level of L3 or L4 vertebra. The ureter exits anteriorly between the cava and the aorta to return to its normal position. The condition is associated with varying degrees of urinary stasis and proximal pyeloureterectasis. The anomaly is due to faulty embryogenesis of the inferior vena cava, with abnormal persistence of the right subcardinal vein anterior to the ureter instead of the right supracardinal vein posterior to the ureter.
Renal Stone Disease
Routine use of noncontrast CT has revolutionized the imaging evaluation of renal stone disease, near completely replacing radiographs and conventional excretory urography in diagnosis of acute ureteral obstruction by renal stones (10,11).
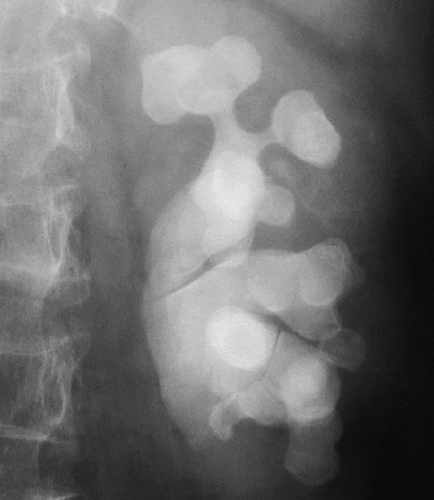
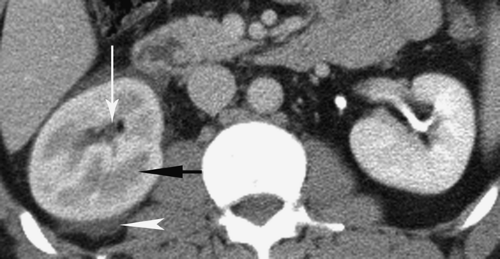
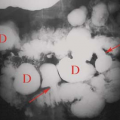
Nephrolithiasis refers to the presence of calculi in the renal collecting system. Nearly 10% of the population will form a renal stone in their lifetime. Sufficient calcium oxalate or calcium phosphate is present in 80% of renal calculi for them to be radiopaque on conventional radiographs. Brushite (2% to 4%) is a unique form of calcium phosphate stones that tends to recur quickly if patients are not treated aggressively. Brushite stones are resistant to treatment with shock wave lithotripsy. Struvite (magnesium ammonium phosphate) stones, formed in the presence of alkaline urine and infection, make up another 5% 15% of renal calculi and are also radiopaque on radiographs. Struvite is the most common component of staghorn calculi (Fig. 33.4). Cystine stones comprise 1% to 2% of renal stones, are mildly radiopaque, and are found only in patients with congenital cystinuria. Uric acid and xanthine stones (5% to 10%) are radiolucent on conventional radiographs. A major advantage of noncontrast CT is that (nearly) all stones are opaque on CT (Table 33.1). The primary limitation of CT is small size of the stone rather than its attenuation. High CT attenuation makes calculi (>200 H) easy to differentiate from other collecting system lesions such as tumors, hematoma, fungus balls, or sloughed papilla, which are all usually lesser than 50 H. Dual-energy CT has been successfully used to determine the chemical composition of stones (12).
Complications of renal calculi include urinary obstruction, ureteral stricture, chronic renal infection, and loss of renal function. Acute flank pain is a common complaint of patients seeking emergency medical treatment. Renal colic, caused by a calculus obstructing the ureter, is the most common cause of acute flank pain and is the usual major consideration for diagnostic imaging. Although most calculi can be detected on plain radiographs, difficulties in localizing the calcification to the ureter and differentiation from other calcifications limit the sensitivity of plain radiography for ureteral stones to as low as 45% with a specificity of only 77%. Noncontrast CT has a sensitivity of 97% and specificity of 96% for ureteral
calculi (10). US has a sensitivity for stone detection of only 24% compared to unenhanced CT (13). An additional advantage of noncontrast CT in the diagnosis of acute flank pain is demonstration of pathology other than a ureteral stone. Among the numerous possibilities are acute appendicitis, incarcerated hernia, ovarian cyst, diverticulitis, and pyelonephritis (14).
calculi (10). US has a sensitivity for stone detection of only 24% compared to unenhanced CT (13). An additional advantage of noncontrast CT in the diagnosis of acute flank pain is demonstration of pathology other than a ureteral stone. Among the numerous possibilities are acute appendicitis, incarcerated hernia, ovarian cyst, diverticulitis, and pyelonephritis (14).
Table 33.1 Urinary Tract Stones | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
Noncontrast renal stone CT is an MDCT scan of the urinary tract performed without oral or IV contrast to detect obstructing ureteral stones and to document stone burden (Fig. 33.5). The thin slice (∼1 mm) capability of MDCT is optimal for this indication. Nearly all stones are visible on unenhanced CT as high attenuation (>200 H), geometric or oval, opaque objects (Table 33.1). Stones appear as white dots on CT scans displayed with soft tissue window settings. The single exception is the soft tissue attenuation (15 to 30 H) crystalline calculus associated with treatment of HIV patients with the antiretroviral drug, indinavir. These calculi may cause ureteral obstruction but should be considered only in this very limited clinical circumstance.
The classic appearance of ureterolithiasis is a high-attenuation stone within the ureter associated with proximal dilatation and distal contraction of the ureter. A halo of soft tissue surrounding the calculus (the tissue rim sign) confirms the stone location within the ureter (10,11). Findings of ureteral obstruction include (1) mild dilatation of the pelvicalyceal system and ureter (>3 mm) proximal to the stone, (2) slight decrease in attenuation of the affected kidney caused by edema; and (3) perinephric soft tissue stranding representing edema in the perinephric and periureteral fat. Sagittal and coronal reformatted images assist in confirming the diagnosis. Focal perinephric fluid collections represent forniceal rupture caused by high-grade obstruction coupled with high urine output. Pitfalls in diagnosis include (1) peripelvic cysts or extrarenal pelvis simulating hydronephrosis; (2) preexisting stranding in the perinephric fat caused by previous inflammation, especially common in older patients; (3) atherosclerotic calcifications; (4) recent stone passage without a stone currently present; and (5) phleboliths. Phleboliths are calcifications within thrombosed veins, particularly common in the pelvis. Differentiation from stones is made by (1) location not along the course of the ureter; (2) absence of a tissue rim sign; (3) presence of a tail sign, a tubular tail extending from the calcification representing a thrombosed vein; and (4) relatively low attenuation of phleboliths with a mean value of 160 H. The probability that a calcification represents a phlebolith is less than 3% when the attenuation is greater than 300 H. High attenuation in the renal pyramids is a sign of dehydration and must not be mistaken for stones. Stones less than 6 mm in size are likely to pass spontaneously through the ureter within 6 weeks. Stones larger than 6 mm are more likely to remain lodged in the ureter and require intervention for removal. Calculi are most likely to be found at the three points of ureteral narrowing previously described.
Hydronephrosis
Hydronephrosis is defined as dilatation of the upper urinary tract. Hydronephrosis is not synonymous with obstruction but has a number of causes that are reviewed in this section. The terms caliectasis, pyelectasis, and ureterectasis are more precise in describing dilatation of portions of the urinary tract. US is an excellent screening modality for determining the presence of urinary tract dilation.
Peripelvic cysts mimic hydronephrosis on noncontrast CT, MR, and on US (15). These are multiple or multilobulated cysts that occupy the renal sinus. They contain clear fluid and may be lymphatic or posttraumatic in origin. Because of the tight confines of the renal sinus as they enlarge, they develop rounded projections that resemble pyelectasis and caliectasis. On CT, they are low attenuation similar to urine. On MR, the fluid is low signal on T1WI and high signal on T2WI. On US, they are thin-walled and anechoic (see Fig 35.52). With contrast filling of the collecting structures, the diagnosis becomes obvious. The enhanced collecting systems are stretched, narrowed, and displaced by the renal sinus cystic mass. Peripelvic cysts are asymptomatic and require no follow-up.
Obstruction. The causes of obstruction include stone, stricture, tumor, and extrinsic compression. The degree of dilatation produced by obstruction is variable. In general, the more proximal and the more chronic the obstruction, the greater is the degree of dilatation (Fig. 33.6). Acute obstruction produced by an impacted stone often produces minimal dilatation. US demonstrates hydronephrosis as separation of normal sinus echogenicity by anechoic urine in the collecting system. The calyces become enlarged and blunted and are seen to connect with the dilated renal pelvis. Medullary pyramids may be hypoechoic, especially in children, and must be differentiated from dilated calyces. Pyramids are more peripheral, surrounded by more echogenic cortex, and do not connect with the renal pelvis. Post-contrast MDCT signs of obstruction
include (Fig. 33.7): (1) increasingly dense nephrogram with time, (2) delay in appearance of contrast in the collecting system, and (3) dilated pelvicalyceal system and ureter to the point of obstruction. Pyelosinus reflux may result from rupture of a fornix precipitated by contrast-induced diuresis superimposed on the increased hydrostatic pressure of an obstructed pelvicalyceal system. Urine and contrast extravasate into the renal sinus and perirenal space. Delay in opacification of the obstructed kidney and dependent layering of unopacified urine over heavier contrast media may also be evident. The location and cause of obstruction can usually be identified (Fig. 33.8).
include (Fig. 33.7): (1) increasingly dense nephrogram with time, (2) delay in appearance of contrast in the collecting system, and (3) dilated pelvicalyceal system and ureter to the point of obstruction. Pyelosinus reflux may result from rupture of a fornix precipitated by contrast-induced diuresis superimposed on the increased hydrostatic pressure of an obstructed pelvicalyceal system. Urine and contrast extravasate into the renal sinus and perirenal space. Delay in opacification of the obstructed kidney and dependent layering of unopacified urine over heavier contrast media may also be evident. The location and cause of obstruction can usually be identified (Fig. 33.8).
Pyonephrosis refers to infection in an obstructed kidney (16). Pyonephrosis can result in rapid destruction of the renal parenchyma and must be treated promptly by relief of obstruction by ureteral stent or nephrostomy tube placement and antibiotics. US classically demonstrates a dilated collecting system filled with layering echogenic pus and debris. Shadowing calculi may also be evident. CT is better than US in demonstrating the site and cause of obstruction. CT demonstrates thickening (>2 mm) of the wall of the renal collecting system and urine of higher than normal attenuation reflecting the presence of pus.
Vesicoureteral reflux is a common cause of hydronephrosis in children (8). The basic defect is an abnormal ureteral tunnel at the UVJ and associated urinary tract infection allowing infected urine from the bladder to reflux up the ureter. In adults, vesicoureteral reflux is usually associated with neurogenic bladder or bladder outlet obstruction. Chronic vesicoureteral reflux of infected urine to the level of the kidney causes reflux nephropathy. Vesicoureteral reflux is confirmed by demonstrating retrograde filling of the ureters on voiding cystourethrography or radionuclide cystography.
Congenital megaureter is due to an aperistaltic segment of the lower ureter 5 to 40 mm in length causing a functional obstruction and resulting in dilatation of the proximal ureter. Ureteral dilatation exceeds 7 mm. The aperistaltic segment of the ureter demonstrates smoothly tapered narrowing without evidence of mechanical obstruction.
Prune belly syndrome, also called Eagle–Barrett syndrome, is a congenital disorder manifest by absence of the abdominal wall musculature, urinary tract anomalies, and cryptorchidism (8). Nearly all patients are males. The ureters are markedly dilated and tortuous, the bladder is large and distended, and the posterior urethra is dilated.
Polyuria, associated with acute diuresis and diabetes insipidus, may cause mild to severe hydronephrosis.
Mass or Filling Defect in Pelvicalyceal System or Ureter
Calculi are the most common cause of filling defects in the contrast-filled collecting system or ureter (Fig. 33.8). Most calculi (>85%) are radiopaque on plain radiographs. Noncontrast CT demonstrates all calculi as high-density objects with a CT density of greater than 200 H. The presence of contrast agent in the collecting system commonly obscures detection of calculi on CT. On MR, stones are seen as foci of absent signal within the collecting system.
Blood clots cause nonradiopaque filling defects that can be differentiated from soft tissue tumors by their change in appearance over time. Attenuation values on CT are usually 40 to 80 H (Fig. 33.9) (17).
Transitional cell carcinoma (TCC) accounts for 85% to 90% of all uroepithelial tumors and is the second most common primary renal malignancy (10% of renal malignancies) (17). Most (85%) have a papillary growth pattern that is exophytic, polypoid, and attached to the mucosa by a stalk. These lesions cause a distinct filling defect in the collecting system (Fig. 33.10) or the ureter. A stippled pattern of contrast material within the interstices of the papillary lesion is characteristic. Nonpapillary tumors are nodular or flat and tend to be infiltrating and aggressive. They cause strictures of the collecting system or the ureter rather than a focal mass. Most TCC occur in men (4:1) aged 60 and older. Various chemical agents used in the textile and plastic industries, drugs including cyclophosphamide and phenacetin, chronic urinary stasis (horseshoe kidney), and smoking play a role in the etiology of these tumors. The tumor metastasizes most commonly to regional lymph nodes, liver, lung, and bone. TCC exhibits a strong tendency toward multiplicity. Patients with upper tract TCC have multicentric tumors in 20% to 44% of cases, and those with TCC of the ureter develop bladder TCC in 20% to 37% of cases. Careful evaluation of the entire urinary tract is essential both at initial diagnosis and for follow-up. Standard treatment
of upper tract TCC is total nephroureterectomy and excision of a cuff of the bladder surrounding the ureteral orifice.
of upper tract TCC is total nephroureterectomy and excision of a cuff of the bladder surrounding the ureteral orifice.
CT shows three typical appearances of TCC of the upper urinary tract: (1) focal intraluminal mass (Fig. 33.10), (2) thickening of the wall and narrowing of the lumen of the ureter or the collecting system (Fig. 33.11), and (3) mass infiltrating the renal sinus and the renal parenchyma (Fig. 33.12) (18). Tumors in the ureter show similar findings (Fig. 33.13) but tend to be smaller at presentation because they cause early ureteral obstruction. On unenhanced CT, TCC attenuation is 8 to 30 H appearing slightly hyperdense to urine and slightly hyperdense to unenhanced renal parenchyma (Fig. 33.14). The much lower attenuation of TCC enables clear differentiation from calculi (>200 H) and usually from blood clots (40 to 80 H). Most focal masses are small (5 to 10 mm). Enhancement, usually low grade, following IV contrast administration confirms a neoplasm. Wall thickening and luminal narrowing are usually symmetric and shows low-level enhancement post-contrast. These findings are not specific and may also be seen with stone passage, hemorrhage, or infection. Ureteroscopic-guided biopsy is usually required. The aggressive infiltrative form of TCC in the kidney extends from the renal pelvis infiltrating the renal sinus and the renal parenchyma while preserving the renal contour (Fig. 33.12) (19). CT stages the tumor by demonstration of the extent of the tumor, including invasion of the kidney or the surrounding structures, lymphadenopathy, and distant metastases (Table 33.2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree