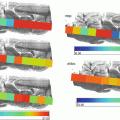
Fig. 1
Chemical structures of nonionic surfactants; (a) n-Octyl ß-d-glucopyranoside (OcGlu), (b) N-Octyl 1-thio-ß-d-glucopyranoside (OcThio), (c) N-Decyl ß-d-maltoside (DDM), (d) N-Octanoyl-N-methylglucamin (MEGA-8)
Concentrations of OcGlu higher than 0.1% (up to 2%) were shown to be compatible with MALDI-MS-based analyses and although this detergent is the most effective for efficient proteolysis , others could be employed with even higher efficiencies and reproducibility such as the nonionic surfactant MEGA-8 [8]. The following protocol provided incorporates the addition of a detergent to trypsin for MALDI-MSI experiments.
2 Materials
All solutions were prepared using ultrapure water (18 MΩ-cm at 25 °C) and analytical grade reagents with a purity of ≥99.5%. Reagents should be disposed of in compliance with health and safety regulations and in accordance with COSHH guidelines.
2.1 Materials for Mass Spectrometry Sample Preparation and Acquisition
- 1.
Ethyl alcohol, 200 proof, ACS reagent ≥99.5%.
- 2.
Ammonium bicarbonate, ≥99.5%.
- 3.
Trypsin Gold, Mass Spectrometry Grade.
- 4.
Octanoyl-N-Methylglucamide (MEGA-8), Anagrade.
- 5.
Octyl-α/β-glucoside 10 mM solution (OcGlu).
- 6.
α-Cyano-4-hydroxycinnamic acid (CHCA), ≥98% powder.
- 7.
Aniline, ACS reagent ≥99.5%.
- 8.
Acetonitrile, for HPLC, ≥99.9%.
- 9.
Methanol, for HPLC, ≥99.9%.
- 10.
Chloroform ≥99.5%.
- 11.
Trifluoroacetic acid, 99%.
- 12.
Phosphorus red, 99%.
- 13.
Aluminium TLC sheets. SILG/UV254 0.20 mm thickness.
- 14.
Extra adhesive precleaned micro slides.
2.2 Reagents: Working Composition
- 1.
Ethanol solutions: Dilute 100% ethanol with ultrapure water to 90% and 70% (vol/vol).
- 2.
Ammonium bicarbonate 50 mM: Dissolve 0.2 g ammonium bicarbonate in 50 ml ultrapure water. Check and adjust pH to 8.0. Solution can be stored for 1 month.
- 3.
Trypsin solution 20 μg ml−1: Add 1 ml ammonium bicarbonate (pH 8.0) directly to the trypsin 100 μg vial to give a 100 μg ml−1 stock solution. Agitate to ensure lyophilized trypsin is reconstituted. For the 20 μg ml−1 working solution; pipette 200 μl of the stock solution into a sterile 1.5 ml snap top Eppendorf and add 800 μl ammonium bicarbonate (pH 8.0).
- 4.
MEGA-8 detergent solution 10 mM: Add 32.142 mg of MEGA-8 to 10 ml ultrapure water. Gently swirl to dissolve taking care not to agitate vigorously. Detergent solution can be stored at +4 °C for 3 months.
- 5.
Trypsin solution 20 μg ml−1 containing detergent 0.5%: Add 5 μl detergent solution (10 mM) to 995 μl trypsin solution 20 μg ml−1.
- 6.
MALDI matrix solution: Prepare 5 mg ml−1 CHCA in 50% (vol/vol) acetonitrile and 0.5% (vol/vol) trifluroacetic acid in ultrapure water. Add equimolar amounts of aniline to the CHCA (i.e., 1 ml of 5 mg ml−1 CHCA solution contained 2.4 μl aniline). Sonicate in a sonic water bath for 5–10 minutes until matrix crystals have fully dissolved. Matrix containing aniline should be prepared fresh on the day of the experiment and not stored.
- 7.
Humidity chamber solution: 50% MeOH in water (vol/vol).
- 8.
Phosphorus red calibrant: 10 mg ml−1 in 100% acetone. Vortex for 10–15 seconds. N.B. phosphorus red may not dissolve entirely.
3 Methods
3.1 In Situ Digestion of Fingermarks
- 1.
Ungroomed fingermarks were prepared and deposited onto aluminum sheets (TLC sheets were pretreated to remove the stationary phase (see Note 1 )).
- 2.
Seven layers of trypsin were deposited at a 1.5 μl/minute flow rate and a nitrogen pressure of 3 bar using the SunCollect pneumatic sprayer (SunChrom GmbH, Freidrichsdorf, Germany) (see Note 2 ).
- 3.
Get Clinical Tree app for offline access
Samples were incubated in a parafilm-covered coplin jar containing the humidity chamber solution; 50% MeOH (Fig. 2), for 3 hours at 37 °C.

Fig. 2
Humidity chamber setup using a glass coplin jar containing 50:50 MeOH: water and a polystyrene floatation device to hold the sample. Once the lid is placed on the jar carefully wrap the seal with parafilm to allow the humidity to remain within the chamber
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree