CHAPTER 22 Percutaneous Biopsy and Drainage
Before the 1980s, open surgery was considered to be the best option for sampling an indeterminate mass or evacuating an infected or symptomatic fluid collection within the abdomen or pelvis. Since then, the development and refinement of percutaneous image-guided techniques have allowed for far less invasive means of accomplishing these objectives. With the proper selection of potential candidates, guidance modality, and approach, image-guided percutaneous techniques have proved safe, efficient, and effective. This chapter discusses some of the considerations and decisions involving percutaneous biopsy and fluid drainage procedures.
PERCUTANEOUS INTERVENTIONS: FOUR STEPS TO SUCCESS
Determine Necessity and Risk
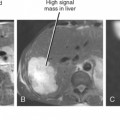
Most image-guided interventions begin with an initial referral from a clinician who does not routinely perform percutaneous procedures. Therefore, one cannot expect the referring physician to be familiar with all of the details regarding the indications, contraindications, techniques, expected outcomes, and complications associated with the requested procedure. Regardless of the opinion or expertise of the referring physician, the radiologist has a responsibility to assess each potential case independently to determine whether the procedure can be performed safely, whether percutaneous access is the preferred approach, and whether the patient is likely to benefit from the procedure (Fig. 22-1). Rare is the referring physician who will stand by your side in court, taking responsibility for the poor outcome of a nonindicated procedure that you performed at their request. In addition, thorough knowledge of the case at hand projects a sense of competence and confidence to the patient, who will appreciate that you took the time to familiarize yourself with their situation. One should only consider percutaneous biopsy of a mass or organ when patient management or treatment decisions depend on definitive characterization of an abnormality that cannot reasonably be characterized noninvasively. Generally accepted indications for percutaneous fluid drainage include suspected infection, need for fluid characterization, or relief of symptoms. Abscess drainage in the setting of cholecystitis, perforated appendicitis, diverticulitis, or Crohn disease may be indicated as a temporizing rather than curative measure.
Choose Target and Approach
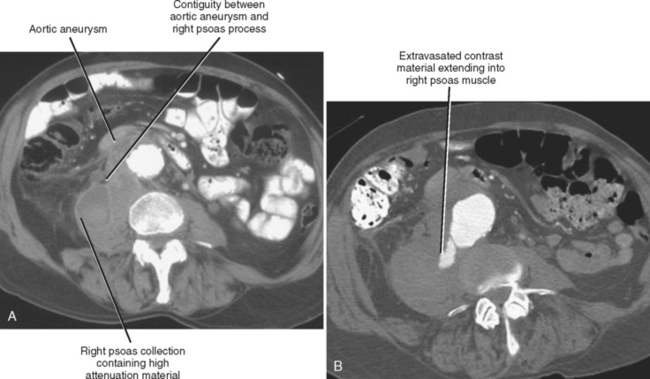
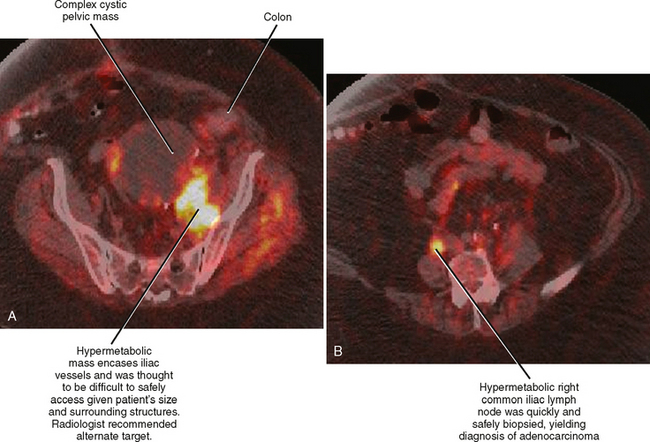
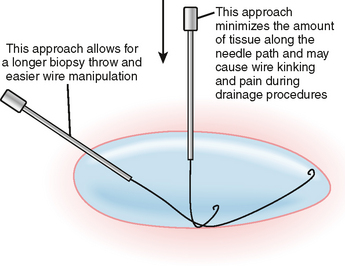
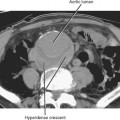
Before performing any biopsy or drainage, one should review all available relevant imaging studies to choose an appropriate target (mass or fluid collection), determine the safest approach to the lesion, and anticipate complications. The most appropriate target for biopsy may not be the lesion initially selected by the referring clinician (Fig. 22-2). Some referring clinicians review diagnostic imaging examinations without ever consulting a radiologist or an official interpretation. Such individuals may refer a patient for biopsy of a particular lesion, unaware that a far more favorable target exists elsewhere in the body. By carefully studying the imaging data in advance, the radiologist can also plan the safest and most direct access to the lesion in question, potentially saving time and consternation during the procedure. Once the access site is chosen, one can estimate the likelihood of certain complications, such as pneumothorax, and appropriately inform the patient.
Choose a Modality
The choice of an appropriate guidance modality (computed tomography [CT], sonography, fluoroscopy, magnetic resonance imaging [MRI]) is important to the success of any percutaneous intervention. When choosing a modality, one must consider such factors as lesion visibility and location, proximity and visibility of surrounding structures, modality availability, and operator experience.
SELECTING A MODALITY
Ultrasound
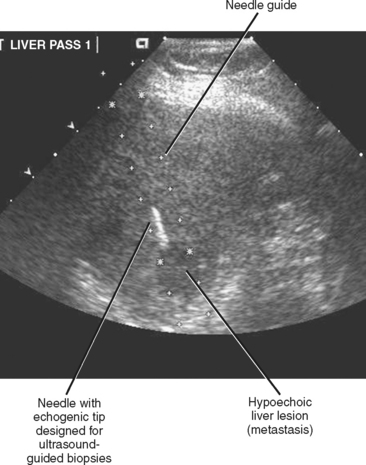
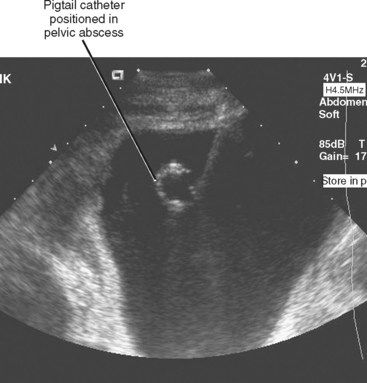
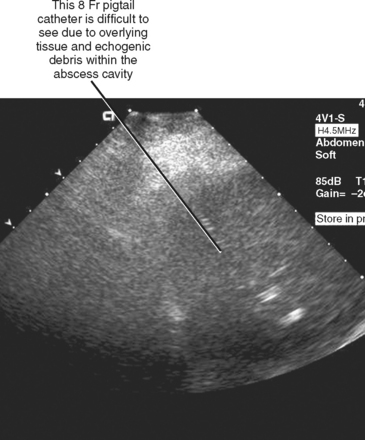
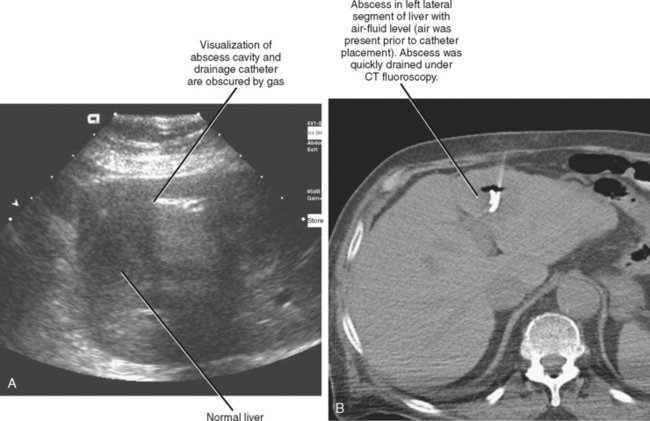
Ultrasound is a popular guidance modality for percutaneous biopsy and abscess drainage (Figs. 22-3 and 22-4). The high temporal resolution of ultrasound facilitates both accuracy and efficiency without subjecting the patient and operator to ionizing radiation. Ultrasound also allows for an unlimited number of imaging planes from which the operator can select the safest projected path. Color Doppler imaging is effective at demonstrating intervening vascular structures, and system portability permits procedures to be performed at the patient’s bedside. Ultrasound is also easy to combine with fluoroscopy. One unique feature of ultrasound is the ability to use it to displace structures such as bowel that lie along the projected needle path. This is accomplished by applying gentle but firm continuous pressure with the transducer over the biopsy site until bowel is displaced to the side. This technique has the added benefit of decreasing the distance from the skin to the target, improving accuracy and target visibility. Unfortunately, the many benefits of sonography are counterbalanced by limited tissue penetration, limited field of view, and the need for an adequate sonographic window. Depending on the background echogenicity, standard needles and catheters may occasionally be difficult to visualize with ultrasound (Fig. 22-5). Visualization of deep structures may be limited in obese patients and in patients with air-filled bowel overlying the region of interest. Air within an abscess cavity may also obscure visualization (Fig. 22-6). The use of tools such as needle guides and echogenic needles designed specifically for ultrasound-guided procedures may improve operator confidence (see Fig. 22-3). If using an adjustable-angle needle guide, one must be certain that the adjustment on the guide matches the angle setting on the ultrasound machine.
Computed Tomography
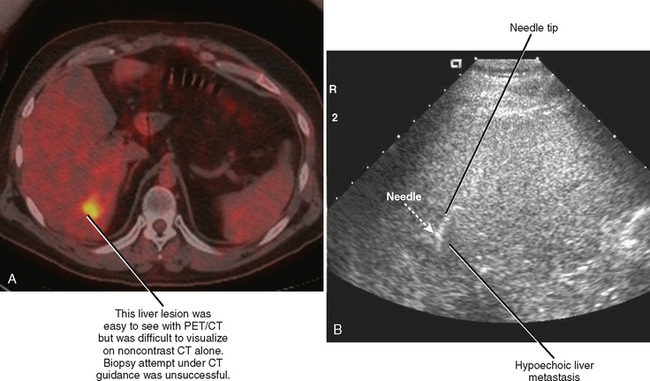
CT provides excellent spatial resolution, field of view, and depth of penetration. However, vascular structures may be difficult to differentiate from surrounding soft tissues on noncontrast CT images, and conventional CT guidance offers relatively poor temporal resolution. Intermittent imaging can be cumbersome for the operator attempting to access a mobile target, and one must rely on estimated trajectories when attempting to avoid nearby structures (Fig. 22-7). Conventional CT imaging is limited to the axial or oblique axial plane (with gantry angulation), lacks portability, and exposes the patient to ionizing radiation.
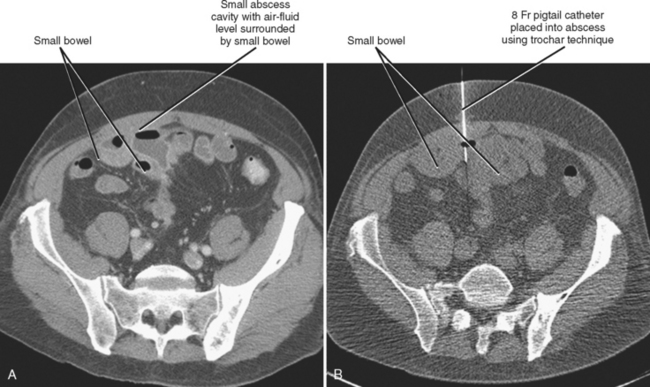
The lack of real-time imaging capability has been perceived as a profound weakness of conventional CT guidance. CT-fluoroscopy units, which became available in the 1990s, improved on conventional CT techniques by providing operator-controlled near–real-time imaging capability. Table and scanner controls, as well as image display, are available in the scanner room to allow efficient operation. Therefore, the operator is exposed to ionizing radiation during CT-fluoroscopy procedures and requires shielding such as a lead apron. Radiation exposure to the operator results from scatter, as well as direct irradiation when the operator’s hands are directly within the beam during needle manipulations. Needle holders have been specifically designed to minimize this latter problem, although traditional surgical instruments may also serve to alleviate exposure of the operator’s hands. In most situations, intermittent monitoring with hands outside the radiation beam is sufficient for needle guidance. Improved efficiency still results from close proximity of the operator to the patient and rapid image acquisition and reconstruction. Because of the potential of CT-fluoroscopy to result in unacceptably high radiation doses to patients and operators, care must be taken to reduce patient exposure (e.g., through modification of tube current and exposure times) and operator exposure (e.g., through appropriate shielding and efficient technique). In addition to improving procedure efficiency, CT fluoroscopy has the potential to improve needle positioning and diagnostic yield of difficult percutaneous biopsies (mobile or small targets), and facilitate safe placement of needles around obstacles such as ribs, bowel, and blood vessels (Figs. 22-8 and 22-9).
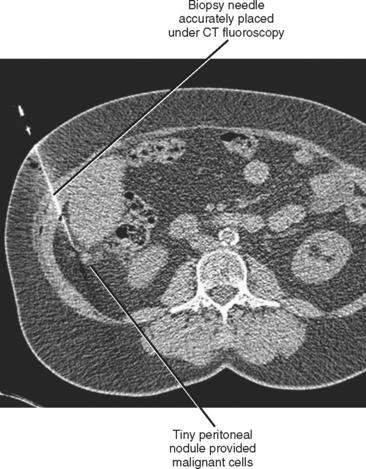
Figure 22-8 A biopsy was successfully performed on tiny peritoneal metastasis using computed tomographic fluoroscopy.
ACCESS CONSIDERATIONS
Preprocedure Access Questions
Potential Approaches
Anterior Transperitoneal (Transabdominal) Approach
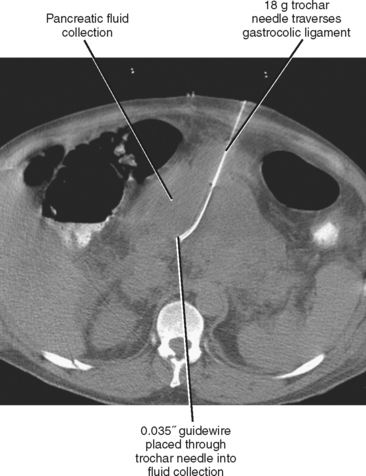
The anterior transperitoneal (transabdominal) approach (Fig. 22-11) allows for comfortable supine positioning of the patient. Peritoneal mesenteries and ligaments (e.g., gastrocolic ligament) are transgressed without difficulty. In thin patients, retroperitoneal structures can be approached in this manner. Bowel creates the greatest impediment to this approach. An effort should be made to avoid the inferior epigastric vessels that run deep to the rectus muscles. Avoiding the rectus muscles by puncturing through an aponeurosis may prevent rectus sheath hematoma. Traversing the peritoneum can be painful for the patient, so care must be taken to provide adequate anesthesia.
Anterior Extraperitoneal Approach
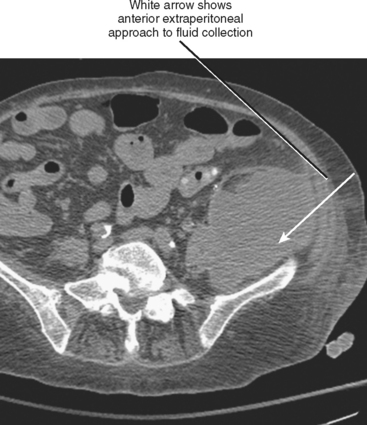
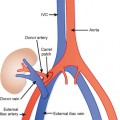
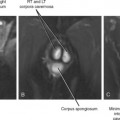
Patients may be positioned supine or lateral decubitus for the anterior extraperitoneal approach (Fig. 22-12), which is useful for biopsy of pelvic lymph nodes and pelvic sidewall lesions, as well as drainage of iliopsoas abscesses. The ureters, external iliac vessels, and deep circumflex iliac arteries are the main structures to avoid with this approach.
Posterior Extraperitoneal (Retroperitoneal) Approach
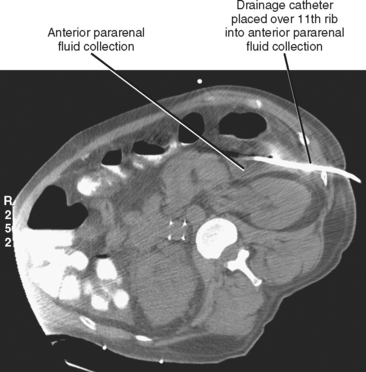
The posterior extraperitoneal (retroperitoneal) approach (Fig. 22-13) usually requires prone or decubitus positioning of the patient but has the advantage of avoiding the intraperitoneal organs. The anterior pararenal approach allows access to many pancreatic fluid collections. A catheter placed in this manner can serve as a guide for a subsequent retroperitoneal surgical approach to the pancreas.
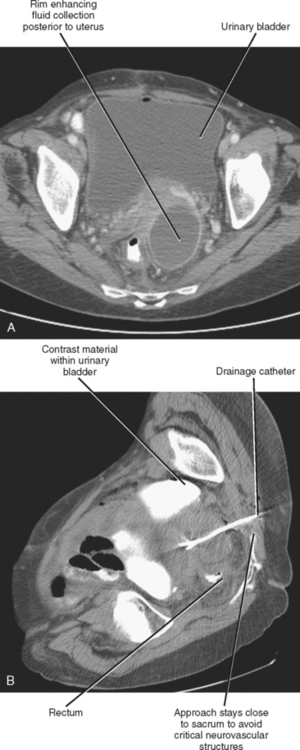
Transgluteal (Transsciatic) Approach
Deep pelvic structures can be approached through the sciatic foramen (Fig. 22-14). Patients are positioned prone or lateral decubitus for this approach. The gluteal vessels and sciatic nerve/sacral plexus are avoided by staying close to the sacrum and below the level of the piriformis muscle. The main limitations of this approach are patient discomfort and limited patient access to the skin entry site for self-care.
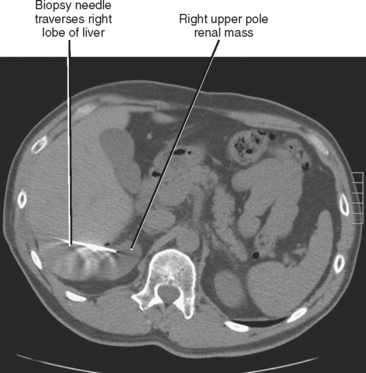
Transhepatic Approach
The liver can be safely traversed when necessary to access fluid collections or biopsy targets within the right adrenal gland, kidney, mesentery, and pancreatic region (Fig. 22-15). An attempt should be made to avoid major hepatic vessels, the gallbladder, and major bile ducts. When placing a transhepatic catheter into an extrahepatic collection, one should try to ensure that no catheter side holes remain within the hepatic parenchyma.